Afamelanotide for Acute Stroke Patients
EXECUTIVE SUMMARY
• World’s first pilot study (CUV801) to evaluate afamelanotide in six arterial Ischaemic stroke (AIS) patients
• Focus on unmet need:
– AIS patients ineligible to receive thrombolytic (IVT, clot dissolving) therapy or endovascular thrombectomy (EVT, mechanical clot removal)
– AIS patients with clots at M2 segment or higher (affecting smaller branches of the main artery of the brain)
• The treatment objectives in CUV801 are to assess:
a. safety of the drug,
b. the size of the Ischaemic penumbra (salvageable Ischaemic brain tissue), and
c. the size of the Ischaemic core (dead brain tissue)
• Efficacy evaluation will be made by comparing imaging and neurological functions (ability to regain lost body functions) over six weeks
CLINUVEL PHARMACEUTICALS LTD today announced that it is evaluating the effects of its drug afamelanotide in patients suffering from arterial ischaemic stroke (AIS) in a pilot study (CUV801). Ischaemic stroke patients suffer a life-threatening condition when a blood clot blocks an artery depriving the brain of blood and oxygen. Afamelanotide will be administered to six AIS patients to evaluate its safety and effectiveness.
ARTERIAL ISCHAEMIC STROKE (AIS)
Stroke patients suffer an acute and life-threatening condition when a clot in a
major brain vessel blocks blood flow and oxygen, causing instant death of
brain tissue. Stroke is often characterised by a patient’s sudden impairment of consciousness, inability to move one side of the body and limitation of speech. Ischaemic stroke accounts for approximately 85% of the estimated 15 million global stroke cases reported annually.
In AIS, the centre of the brain injury is called the necrotic core (dead brain
tissue), and the larger surrounding brain injury is characterised as the
penumbra (literally: shadow around the core). The necrotic core is brain tissue beyond rescue, whereas the penumbra is the part of the brain deprived of oxygen but still salvageable if immediate therapy is administered to the stroke patient.
The current standard therapy relies on early intervention following stroke onset to restore blood flow to the brain by either chemically dissolving or physically removing the clot. Globally, intravenous thrombolytic therapy (IVT) in the form of recombinant tissue plasminogen activator (alteplase or tenecteplase; rt-PA)is administered within 4.5 hours, aiming to dissolve the clot within the brain artery. Estimates vary, but 7-21% of patients presenting to hospital within the therapeutic window receive IVT. This therapy is usually combined with endovascular thrombectomy (EVT) which aims to mechanically remove the clot, preferably performed within 24 hours of the stroke. EVT is a relatively new procedure, only 11% of US stroke patients are considered eligible and approximately 12% of AIS patients receive this therapy in Europe.
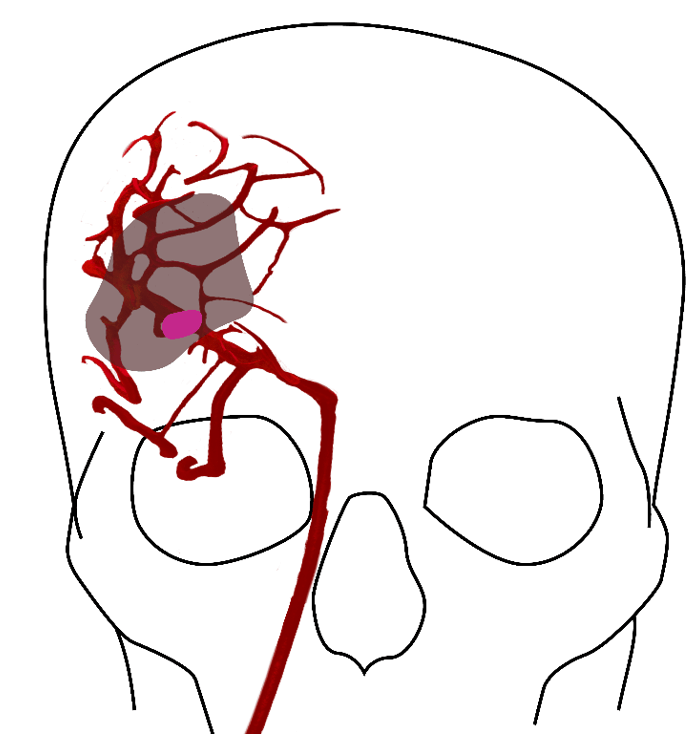
Figure 1: A clot in the brain vessel of a stroke patient leads to instant death of brain tissue closest to the clot, shown in dark pink. The larger area surrounding the core (shaded) is characterised as the penumbra, tissue which can still be rescued.
Brain imaging, using a computed tomography (CT) scan, is performed upon hospital admission for patients with symptoms of brain injury. Physician assessment of the loss of neurological functions is made using the modified Rankin Scale (mRS), a scoring of severity of disability. In Europe, over 85% of AIS cases presenting to hospitals are not eligible for current standard of care treatment.
The location of the brain clot has a direct impact upon the possibility to offer the combined therapy (IVT) and EVT treatment, as well as overall patient prognosis. Clots in the main “M1” segment of the middle cerebral artery (MCA) are generally eligible for IVT or EVT due to the diameter of the artery and the ability to physically remove the clot, whereas those in smaller arteries (segments “M2” and higher) are considered impossible to remove or of too great
a risk to the patient.
AFAMELANOTIDE AND TARGET POPULATION IN AIS
Relevant to planned AIS treatment, afamelanotide is shown to exhibit neuroprotective, vasoactive (acting on blood vessels), anti-oncotic (anti-swelling) and anti-oxidative effects by optimising blood flow, and reducing the size of an injury and fluid (oedema) formation.
Afamelanotide will be administered to those AIS patients who are ineligible to receive standard therapy (IVT/EVT), and in stroke affecting the M2 segment or higher, where there is a great unmet medical need.
STUDY DESIGN – CUV801
The first pilot Phase IIa study (CUV801) of its kind will evaluate SCENESSE® (afamelanotide 16mg) in six AIS patients.
CUV801 will be conducted over the course of six weeks, at a single expert neurological emergency centre, whereby assessments will be made of the brain injury through MRI scans and according to overall disability (neurological assessment, NIHSS7 and mRS8).
The primary study objective is to assess the safety of SCENESSE®, while the secondary objective is to assess whether the therapy affects the size of the penumbra, by increasing blood flow, restoring oxygen supply to the brain, and reducing the amount of cerebral oedema (fluid) which is seen as a result of the stroke. Positive findings would indicate that the drug is able to support brain tissue-at-risk and provide overall neuroprotection and benefit to stroke patients.
CUV801 will commence recruitment once COVID restrictions on available medical staff have eased.
SAFETY AND EFFECTIVENESS OF SCENESSE® (AFAMELANOTIDE 16mg)
Afamelanotide, the active ingredient in SCENESSE® is a tridecapeptide, an analogue of the naturally occurring alphamelanocyte stimulating hormone and belongs to the group of proopiomelanocortins (POMC). As scientifically reported, afamelanotide exerts a number of pharmacological effects which may be of clinical benefit to patients with acute and life-threatening disorders. More than 10,000 doses of afamelanotide have been administered to over 1,400
individuals during its development and use across a period of nearly two decades. The safety profile of afamelanotide as a controlled-release injectable implant formulation (SCENESSE®) has been shown to be consistently positive. SCENESSE® is currently approved in Europe, the USA and Australia for adult patients with the rare genetic disorder erythropoietic protoporphyria.
STROKE PREVALENCE, PROGNOSIS AND IMPACT
Globally, 15 million patients suffer from a stroke annually, and over 5.5 million do not survive. Around 40% of AIS patients do not survive the first year, with as many as 50% of stroke survivors suffering permanent disability. A quarter of stroke patients will experience a second stroke within five years, with 12% of AIS patients being readmitted to hospital within 30 days of discharge. Most patients require ongoing care and unfortunately suffer limitations in their daily social activity and productivity. Families often have to shoulder excessive rehabilitation costs and lost wages. In the United States alone, recent estimates place the cost of stroke in excess of $34 billion per year.
COMMENTARY
“For years we had prepared for the use of afamelanotide in stroke patients, given the broad effectiveness of the hormone and the significant medical need to improve clinical outcomes in these patients,” CLINUVEL’s Chief Scientific Officer, Dr Dennis Wright said. “As with other life-threatening indications, we needed to collect sufficient safety data on afamelanotide before starting this program and now have two decades of data and over four years of post-marketing authorisation experience. We are now in a position to translate the use of afamelanotide in life-threatening disorders. We are starting from a robust foundation of safety, making this translational use possible. “The objective of medical intervention in stroke is to offer a safe therapy for large populations not receiving any at present, by assisting restoration of blood flow and oxygen supply to the brain while minimising fluid accumulation. The human reward for us is to reduce the overall damage stroke causes to those patients,” Dr Wright said.
– End –