PRÉNUMBRA® – New Liquid Afamelanotide Presentation
EXECUTIVE SUMMARY
• PRÉNUMBRA® non-solid dosage form of afamelanotide
• Active life-cycle management of melanocortin-based therapy
• Second formulation of afamelanotide to be used in acute disorders
• Product to be used for haemodynamic, vasoactive, and anti-oncotic potency
• CLINUVEL holds proprietary rights for formulation, identified indications & trademark for PRÉNUMBRA®
CLINUVEL PHARMACEUTICALS LTD today announced its expeditious development of PRÉNUMBRA®, a new nonsolid (liquid) presentation of its drug afamelanotide. PRÉNUMBRA® follows the development of SCENESSE® (afamelanotide 16mg) aimed at allowing dosing flexibility as part of the active life-cycle management of afamelanotide and to address clinical needs in critical disorders. 1 CLINUVEL will be evaluating PRÉNUMBRA® in to-be-announced clinical indications. The Company has been able to secure both the intellectual property rights for the dosage form in the identified indications as well as the international registered trademarks for PRÉNUMBRA®.
LIFE-CYCLE MANAGEMENT OF AFAMELANOTIDE
Afamelanotide is an analogue of alpha-melanocyte stimulating hormone (α-MSH) and belongs to the family of proopiomelanocortins (POMCs). There are five identified melanocortin receptors expressed in various tissues and organs, with afamelanotide exerting a clinical effect through binding to four of these receptors. Among the multitude of pharmacological properties of afamelanotide, it can serve clinically as a non-steroidogenic hormone. Variation in the dose frequency and strength provides clinical potential in the treatment of acute disorders. PRÉNUMBRA® is the second formulation of afamelanotide to be progressed through an expedited clinical development program by CLINUVEL. The Company has made available SCENESSE®, a novel controlled-release injectable implant formulation of afamelanotide, in the USA, Europe and China for adult patients with the rare genetic disorder erythropoietic protoporphyria (EPP). SCENESSE® is also being evaluated as a therapy for systemic photoprotection, systemic repigmentation and DNA repair in further clinical indications. From the pharmacological mode of action of afamelanotide a positive safety profile and clinical effectiveness have been established over more than 15 years of clinical development and use under real-world conditions; in total, in excess of 10,000 afamelanotide doses have been administered in more than 1,400 individuals, with a cohort of EPP patients in Europe receiving annual treatment for over 10 years.
PHARMACEUTICS OF AFAMELANOTIDE
In managing the life cycle of afamelanotide, a synchronous strategy has been followed to generate pharmacological data in pre-clinical models and human subjects, analysing dose intervals, dose levels, formulations, and biological effects (the body’s response to the drug). In total four formulations, three dose intervals, and four dose strengths had been evaluated over more than a decade, which resulted in the first product SCENESSE®, now distributed as a solid dosage form administered to EPP patients every two months. Originating from CLINUVEL’s evaluation of scientific data, PRÉNUMBRA® is a liquid presentation delivering afamelanotide systemically, allowing flexible drug loading in a number of patient populations where benefit is expected from a different dose and dose frequency than is currently seen from the SCENESSE® injectable implant formulation. The addition of PRÉNUMBRA® is part of the organic expansion of CLINUVEL’s product pipeline.
APPLICABILITY OF THE NEW FORMULATION
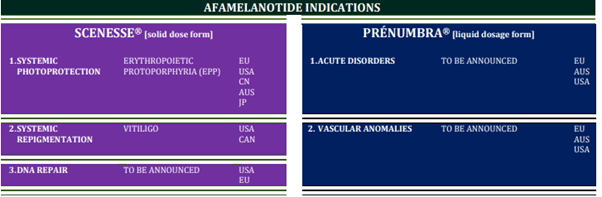
CLINUVEL is advancing PRÉNUMBRA® as a potent haemodynamic, vasoactive (acting on blood vessels) and antioncotic (counteracting fluid formation in tissues) therapeutic agent, initially in adult patients. PRÉNUMBRA® will be evaluated in a number of critical disorders with vascular anomalies and oedema (fluid formation) – where corticosteroids and other anti-inflammatory drugs have not been successful, cause drug dependency or severe side effects. The new medical applications will be disclosed when the respective ethics committees and regulatory authorities have agreed to the expanded use of afamelanotide. As an overview, the various indications for both dosage forms of afamelanotide are illustrated below.
COMMENTARY
“The foundation of our enterprise has been, and will also always be, our approach to the toxicology and safety of SCENESSE® and the way we make new products clinically available,” CLINUVEL’s Chief Scientific Officer, Dr Dennis Wright said. “In developing SCENESSE® we kept our focus on new formulations and we saw the advent of an exciting phase with the development of PRÉNUMBRA®. This first of the new formulations provides us with flexible treatment options for identified patient groups beyond and quite distinct from those we are already addressing with SCENESSE®. The two products will complement each other and address various acute and life-threatening diseases. “We had earmarked the use of afamelanotide as an anti-oedematous drug since the molecular properties and pharmacology lend themselves to the intended uses. However, we first needed to safely progress afamelanotide as a systemic photoprotective and repigmentation agent in Europe and North America before taking the regulatory authorities along to the wider use of the molecule. We have a growing body of data on the long-term use and safety profile of SCENESSE® and this gives comfort as we engage regulatory authorities on the use of PRÉNUMBRA® in the clinic. “We have, for some time, prepared to commence clinical studies this calendar year, engaging with regulatory authorities and through an accelerated pathway will seek authorisation for PRÉNUMBRA® in globalmarkets. The first non-solid dose product will be made available for adult patients and, with the collection of sufficient safety data, a presentation suitable for paediatric patients will then follow. “Our overall aim of expanding the use of melanocortins will turn to reality by providing clinicians with more than one therapeutic option to treat critical conditions. There is strong global regulatory support to address unmet clinical needs with novel formulations, and CLINUVEL’s team is well placed to pursue these opportunities,” Dr Wright said.