First patient dosed in SCENESSE® DNA Repair Program
Xeroderma pigmentosum (XP) patient receives SCENESSE® treatment
CLINUVEL PHARMACEUTICALS LTD today announced that the first patient diagnosed with xeroderma pigmentosum (XP-C) has received SCENESSE® (afamelanotide 16mg).1 The Special Access Program of one patient is part of CLINUVEL’s DNA Repair Program.
CLINUVEL is evaluating SCENESSE® in a staged clinical program to confirm the drug’s ability to assist repair of ultraviolet (UV)-induced DNA damage of skin. SCENESSE® is currently prescribed as standard of care for erythropoietic protoporphyria (EPP) in the USA and Europe.
SCENESSE® IN XERODERMA PIGMENTOSUM
The first XP-C patient serves to confirm the safety of the product. Given the high mortality rate in XP-C, the safety of afamelanotide will be evaluated during 42 days of treatment. After confirmation of the safety of the drug product,the DNA Repair Program will proceed with an open label Phase II study involving six XP-C patients (CUV150) and a control study enrolling 10 healthy volunteers (CUV151) whereby clinical and histological (skin biopsies) evaluation of afamelanotide treatment will be undertaken in both groups.
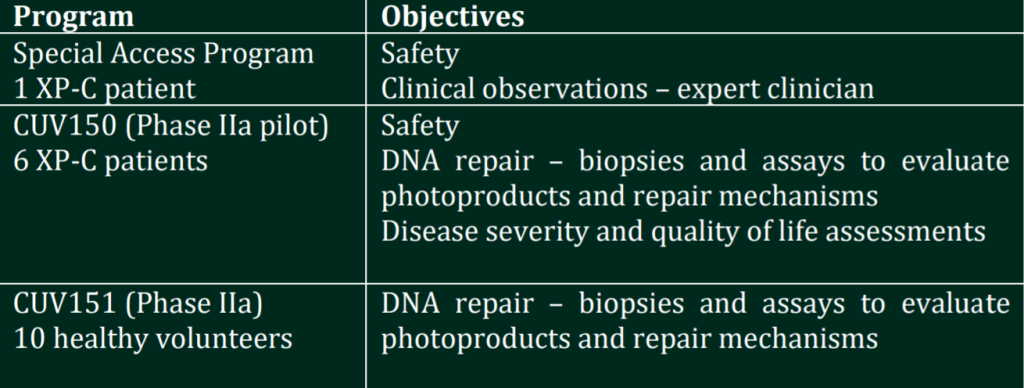
Details of the sponsored studies CUV150 and CUV151 will be released in the following weeks.
SCENESSE® DNA Repair Program
Scientific evidence supports the use of afamelanotide, the active ingredient in SCENESSE®, to protect skin from UV and light (systemic photoprotection), and repair UV-induced DNA damage. As part of a staged clinical program, CLINUVEL has worked with global experts to identify and design clinical objectives which focus on clinically meaningful and relevant study endpoints, including assessment of DNA photoproducts (chemical reactions within DNA strands), repair of DNA damage, and evaluation of the treatment impact on patients’ quality of life.
Xeroderma Pigmentosum (XP) patients incur accelerated DNA damage
Ultraviolet (UVB of wavelengths 290-320 nm and UVA of 320-400 nm) and high energy visible (HEV, 400-600 nm) light penetrate human skin leading to oxidative stress and damage to the DNA helix within the nucleus of skin cells. The photodamage consists of loss of connective tissues and changes to the DNA strands (generation of photoproducts). XP patients exhibit extreme deficiency in repair of UV-provoked DNA damage, characterised as a model for the most severe form of photodamage. XP has a high rate of mortality with a median survival of 30 years and affects approximately 1:450,000 individuals in the European population. XP has eight variants (XP-A to G, and V), reflecting eight different genes involved. Each XP gene is involved in nucleotide excision repair (NER), a process of identifying, “snipping”, removing and replacing damaged sequences in DNA. If left unrepaired, damaged DNA can replicate and increase the risk of skin cancers, including melanoma. Due to the inability to initiate or complete the NER process, XP patients are at 10,000- and 2,000-fold risk of nonmelanoma and melanoma skin cancers, respectively. Most XP patients will experience the first skin cancer before adolescence, while the leading cause of death remains progressive non-melanoma skin cancers and melanoma in the third decade. Due to the extreme rate of these malignancies, surgical intervention is required frequently, resulting in loss of extremities, facial anatomy such as ears, and eyesight.
Commentary
“Having recently announced the scope and aims of the staged DNA Repair Program, it is important to see the first XP patient treated under the Special Access Program,” CLINUVEL’s Chief Scientific Officer, Dr Dennis Wright said. “We have worked closely with the expert clinic to facilitate this first patient’s treatment and we await feedback on the response. “The use of SCENESSE® in XP, being a model studied for DNA repair originating from UV exposure, is exciting and represents a potential breakthrough for individuals at higher risk of photodamage. Our team has methodically worked towards this one objective and will be the first company to make the final clinical link between afamelanotide and reduction of the risk of skin cancers by addressing DNA damage caused by UV exposure. The thorough work towards the safety profile of SCENESSE® gives us comfort to finally evaluate its use in this group of patients,” Dr Wright said. “We seek to provide meaningful benefit to XP patients, and these results will serve a wider population of fairskinned individuals at risk of developing skin cancers,” CLINUVEL’s Clinical Operations Manager, Dr Pilar Bilbao said. “The next 12 months will be exciting for many patients, their families, the clinical experts and our own teams.”
1 SCENESSE® (afamelanotide 16mg) is approved in the European Union as an orphan medicinal product for the prevention of
phototoxicity in adult patients with erythropoietic protoporphyria (EPP). SCENESSE® is approved in the USA to increase “painfree”
light exposure in adult EPP patients with a history of phototoxicity. Information on the product can be found on CLINUVEL’s website at www.clinuvel.com.
Download PDF