CLINUVEL Newsletter VI
Dear shareholders, friends,
CLINUVEL’S OUTLOOK
The past weeks have seen a flurry of activities which call for much more narrative to do justice to the news flow.
One, the Strategic Update lodged on 29 October 2020 illustrated the internal restructuring of the Group over three divisions, Pharmaceuticals, Healthcare Solutions, and Communications, Branding and Marketing. The restructure had been anticipated for some time, and COVID played a part of when to implement. With the restructuring, new talent and professionals with specialised skills are joining the team. I see innovation as the only guarantee for value long-term, so also for CLINUVEL.
So, let us walk through this update part by part. First CLINUVEL’s core business centres around pharmaceuticals in its widest possible form, new molecules, formulations and indications. To implement a strategy of five products and a minimum of five indications requires a dedicated team led by managers who commit longer term. An example is VALLAURIX Singapore as the Research, Development, and Innovation (RDI) Centre. RDI staff are focussed on a comprehensive line of pharmaceutical and OTC products which is clearly pivotal to our expansion plans. Dr Wright is overseeing the RDI expansion and programs, while Drs Rai and Zhao and Mr Choy are responsible for day-to-day operations and output from the teams on the ground.
In our clinical diversification, the team led by Dr Bilbao has made strides towards the stroke program (arterial ischaemic stroke, AIS) and DNA Repair Program comprising xeroderma pigmentosum (XP) and healthy individuals prone to UV-induced skin damage. In 2021, there is much more expected from her team in terms of innovative direction and read outs (results).
With the expansion strategy comes a diversification into wider non-prescription markets for which CUV will have a number of over the counter products assisting wider audiences in DNA repair, protection and dermocosmetic care.
The expansion of the Group has called for the new Communications, Branding and Marketing Division which will actively engage with an online audience to grow the visibility of CLINUVEL’s causes. In the wake of these activities, we will establish an Agency style team which will be able to offer services and content, as well as produce multimedia fitting within modern marketing. The aim is to generate a much higher visibility to the Group and its core activities.
In the restructuring of the Group along three divisions but working cross-functionally, the entire Company will be aligned in its annual and long-term objectives. My expectation is that the conglomerate of all activities will provide broader public awareness of CLINUVEL long-term.
DNA REPAIR PROGRAM
The DNA Repair Program is progressing since it has been confirmed that the first XP-C patient has completed the treatment and has not suffered any adverse reactions to SCENESSE® (afamelanotide 16mg).1 The first and important step has been taken in proving safety beyond reasonable doubt in a patient population developing recurrent skin cancers necessitating mutilating surgeries. The second objective is to get the CUV150 study under way and evaluate the effects of afamelanotide in DNA-regeneration, skin damage and overall well-being in XP-C patients. With much anticipation and somehow higher expectations than any other study, we look forward to the first results in 2021.
STROKE PROGRAM (AIS)
The stroke program has received many questions and vocal support, often coming from long standing shareholders with considerable surprise. Perhaps a few words to our purpose and long preparation.
The use of afamelanotide and our follow-on molecules was planned in 2010, whereby we laid out a program to complete the erythropoietic protoporphyria (EPP) trajectory before venturing out to other indications. Safety and commercial viability of the product SCENESSE® were major drivers in our expansion strategy.
The extent to which SCENESSE® would elicit the desired central effects hinged on a number of pharmacological parameters and assurance on safety. Passing the four-year commercial distribution mark, all possible obstacles to start our stroke program had been mastered; the further rationale and technical arguments can be read from the transcripts of our Strategic Update.
The translational use of our hormone in stroke is quite a therapeutic distance from CLINUVEL’s work to date, but as we have stressed at length, the strict vigilance on safety of this product over the years has been the principal requisite to further use the product in life threatening disorders. We always knew that without this attention to safety, we could not have advanced in XP, stroke patients, or successfully develop our melanocortin derivatives; along this course our strategy is unfolding according to plan.
In the stroke program, CLINUVEL will evaluate the effects of afamelanotide on the ischaemic core (the dead parts of the brain) and the penumbra, the oxygen deprived parts of the brain which can be rescued if blood flow is restored. For this the evaluation of the various bran imaging performed in patients will give a swift answer as to any effect seen.
Our clinical team is working closely with the leading neurologists and neuroradiologists (specialists in brain imaging).
DIVERSIFICATION MELANOCORTINS
The frequent discussion, and also one initiated by our analysts, centres around the mode of action and scope of afamelanotide and melanocortins.
The best way to describe afamelanotide’s mode of action is by viewing it as a molecule belonging to, and affecting, the hormonal axis (hypothalamus-pituitary gland-adrenal gland, HPA). We possess a hormone which essentially restores balance (homeostasis) where tissue damage and oxidative damage takes place following sterile, non-infectious conditions.
In circulatory ischaemic events the use of afamelanotide and follow-on molecules is best at place. In simpler terms, we start to deploy a family of hormones without the adrenal effects (non-steroidogenic). The afamelanotide hormone offers ample pharmacological application within limits; we do not possess an omnipotent ubiquitous agent, but one which requires care and thought as to which patient populations to target and where to refrain from use.
All that said, it is a rare privilege to be able to unfold a strategy which had been carefully planned and which awaited various technical milestones along a course of decades. During this time, the scientific community has recognised the potential of CLINUVEL’s work, as more melanocortins are being developed by other companies for other indications. More will come from us in 2021.
FINANCIAL MANAGEMENT AND COVID
As we are wading through the consequences of the COVID year, some questions have reached us of how CLINUVEL will prepare for a possible third wave and longer restrictions. Against the spread of viral load in Europe and the United States, the promise of widespread vaccination is a challenge to the already stretched resources of hospitals since other patients deserve diagnosis and ongoing treatment. In the past two News Communiqués (IV and V), I have elaborated on our financial strategy in order to attenuate future unforeseen risks. In hedging against tail risks of catastrophic unforeseen events, we have made clear that a successful and sustainable pharmaceutical company should have two to three years of liquidity acting as a buffer to global commercial uncertainty. With the recent financials released on 29 October (quarter ending 30 September), it should become apparent that CLINUVEL is gradually approaching the self-imposed financial objectives benefiting all stakeholders. In setting financial standards high, our financial officer signals to us that the finance team foresees a volatile environment ahead and acts in anticipation of these times. I see CLINUVEL’s current success as a function of our ability to prepare for so-called inconceivable, black swan, events.
This is not to imply that we hold a grim view of CLINUVEL or the world, it simply states that we use our broad experience in reading markets, economic conditions, and setting up the Company in anticipation of crises and recessions.
There are too many lessons to be learned in managing public companies, and no contraction is triggered by the one or the same event, however the importance of cash flow and the role of a safe level of leverage are recurring themes which occupy us. In looking at CLINUVEL’s historical current ratios ranging from 4.5 times in the mid-2000’s to around 12 times more recently, and cash reserves at any time – always above A$5 million, or six months of runway, during the development stages – one can discern a red line of how this team has approached the business in the early years. This past behaviour is an indication of how we will manage the business moving forward. Naturally, one can always issue more equity at reasonable discount when the need for cash is the highest, but I have expressed my views on this phenomenon more than once.
CLINUVEL’s financial position is continuing to strengthen. As I stated in News Communiqué V, there is no coincidence underlying our position, nor do we take this status for granted: every day at work is one when we fight for our patients’ causes and by no means does it come easily. Our cash position allows us to take exhaustive steps to reach our corporate goals, litigate justifiably when needed, and expand the Company with specific and required talent. All professionals, young and mature, fully understand that we need to meet series of objectives within 21 months and that our current success is just a transition to a larger diversified entity pursuing more success.
USA DISTRIBUTON UPDATE
It has been a challenging yet productive year as the US team efficiently identified and issued training programs and accreditation to 34 EPP Specialty Centers across the US. It is expected that a few more will join the pool of prescribing Centers. The US team continues to evaluate and identify Centers within the clusters of the EPP patients to facilitate treatment access and reduce travel time for these patients.
As stated, Dr Teng and her team are 19 months ahead of schedule and have performed well. Our strategy needed to change dramatically following the outbreak of COVID-19 in February and this team showed the flexibility to adapt when it was required. Above all the US team shows proactivity in assisting US EPP patients with the insurance applications in obtaining Prior Authorization.
The EPP patients have been understanding regarding the process of Prior Authorization in the midst of the pandemic. They have expressed their appreciation to the US team in making their treatment a priority. It has been an honour and a pleasure assisting the EPP patients during this journey.
Over 60 insurers are covering the SCENESSE® treatment and several large institutions across the US have approved SCENESSE® for addition to their pharmacy formulary.
The feedback on efficacy of treatment is unchanged and patients report “life-changing” therapy enabling them to undertake activities and participate to full life, new experiences deemed impossible before. More clinical and post-marketing activities are planned in the US which will serve our patient population, while the Savings Programs is under way.
Pleasingly and after much work, a new Level II HCPCS unique J-code (J7352) for SCENESSE® as “Afamelanotide implant, 16 mg” has been assigned by the HCPCS (Healthcare Common Procedure Coding System). The HCPCS codes provides a standardised coding system for describing specific items and services and are primarily used for billing and identifying items and services. This code will be effective as of 1 January 2021 for use on insurance claims with dates of service on or after 1 January 2021.
PUBLIC AND INVESTOR RELATIONS
Since the last Communiqué, the brisk pace of the Company’s announcements has continued.
The 2020 Annual Report and Notice of Meeting for the 11 November 2020 Annual General Meeting were issued in October. Many compliments were received on the readability and informative coverage of strategy, the rationale for focus on DNA repair, and the Company’s progress and achievements during the 2019/20 financial year. The theme of growth and expansion has been well appreciated.
Three presentations were made to conferences across October and November, taking the CLINUVEL story to many new potential investors. The commencement of new independent coverage of CLINUVEL by Jefferies Equities Research, was a welcome expansion of the coverage of CLINUVEL – refer to the analyst coverage page of our website.
In October, we announced the extension of SCENESSE® to a new jurisdiction and progress in new indications. The safety reports on the first treatment of an XP-C patient with SCENESSE®, part of our DNA Repair Program, were positive. In Australia, SCENESSE® was approved by the Therapeutic Goods Administration (TGA) for the prevention of phototoxicity in adult patients with EPP and we remain committed to facilitate treatment access to SCENESSE® for EPP patients worldwide. A new indication, AIS, was announced and we continue to work toward commencement of a Phase II clinical study to assess the safety and efficacy of SCENESSE® to provide a therapy for the large number of stroke patients whose condition is currently untreatable.
Cash receipts for the September quarter 2020 and a comprehensive strategic update were also announced in October. Cash receipts from distribution of SCENESSE® in Europe and the USA were A$12.015 million. The dividend was made to shareholders in October (of A$1.235 million).
The Strategic Update has been reiterated in the CEO’s address to shareholders on 11 November and the corporate presentation to the Jefferies Healthcare Conference on 19 November. The organisation of the CLINUVEL Group into three divisions, underpinned by the RDI Centre in Singapore has been well received. The expansion of indications being progressed and the formation of the Healthcare Solutions Division with anticipation of the first over-the-counter product in 2021 has excited shareholders. It is evident that it will take some time for the market to digest the growth and diversification of CLINUVEL. The new Communications, Branding and Marketing Division will be driven to increase awareness amongst targeted audiences.
In November, we held the Annual General Meeting, and this is commented on separately below. The Chair’s Letter of 19 November was the third for the year and provided an optimistic assessment of CLINUVEL’s outlook. Together with this Communiqué, the sixth for the year, we continue the peer leading practice of regular and direct communication with shareholders from the desk of the Chairman and the CEO.
All the announcements of the Company are available on the CLINUVEL website. The key announcements for 2020 are listed in the table below for ease of reference.
The Annual General Meeting (AGM)
The 2020 virtual AGM on 11 November was attended by more shareholders than the 2019 AGM which itself was better attended than previous years. However, CUV currently has over 6,100 registered shareholders and understands the vast majority are highly supportive of the direction and performance but tend not to engage on Company issues. We would like to see more CUV holders attend future meetings.
The Chair’s address provided insight to the low vote cast by shareholders for the Renumeration Report in relation to the shares on issue and the impact on the outcome of the votes against the resolution which accounted for 11.8% of all shares on issue. We will communicate to shareholders over the coming months and provide more content on the Board’s approach to remuneration of key executives, which it believes is appropriately structured to incentivise the peer leading performance of our Company. We will also brief proxy advisors, so their analysis is more rigorous, particularly when making peer group comparisons.
Whilst there were some technology glitches during the virtual meeting, persistence resulted in a productive meeting, with the CEO’s presentation on the strategic path of CLINUVEL and Mrs Anne Wilson’s testimony on how she has managed EPP and what the TGA approval means to her, struck a chord with many shareholders. A copy of the AGM webcast is available on the Investor section of our website.
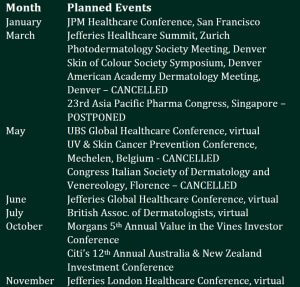
Calendar of planned events
The calendar of planned corporate events was affected during the year by postponements and cancellations due to the coronavirus pandemic.
We managed to present to several conferences, mainly virtually, during the year. We thus reached many new potential investors to tell the CLINUVEL story and the compelling investment rationale. Corporate presentations are posted to the ASX when they contain new content and previously unannounced news, and this keeps all stakeholders informed of the progress of the Company.
SUMMARY
In February 2020, I held no high hopes that CLINUVEL would navigate the global pandemic unscathed. However, as Charles Dickens mused his opus, I look back at a year where our and my team have delivered Great Expectations. Under guidance of Mr Lachlan Hay, Dr Azza Hamila and Mrs Colucci European distribution was managed beyond what can be expected, Scientific progress has been booked by Dr Wright and Dr Rai and the Singaporean and Australian teams led by Dr Zhao. Mr Bull worked tirelessly with new shareholders and institutions and in physical isolation our output was actually higher than in previous years, Our CFO and finance team worked at full capacity keeping us within budgetary limits, all in all a great year under exceptional circumstances, All managers and supporting staff deserve applause for what they have performed this year, many many thanks. Most pleasing is seeing young talent picking up the baton and finding solutions where others could not, it is a privilege to see the next generation of talent making their mark on our company.
As we worked towards the end of the calendar year, on 8 October we quietly celebrated the first anniversary of the US Food and Drug Administration (FDA) marketing authorization for SCENESSE®. The Company has come a long way, but it is perhaps best to state that we are just starting our journey. We needed to overcome all global regulatory, local ethics and national hurdles to unfold the technologies. The 15 previous years have enabled us to play out our strategy, but we are just at the beginning of much more to follow. The Company is managing a once-in-a-generation overall program, and we have adapted to a transdisciplinary approach to advance our individual projects and products.
The COVID world forces creativity and we use all our skills in these difficult times as many millions have lost employment. Under these circumstances, I see that our staff have learned to appreciate what they hold and the environment they have created since the world has been in a state of incertitude and trepidation.
I daily witness the enthusiasm within the Company, with various of our key projects moving along synchronously. Our front and back office staff are well aligned to see through our next set of objectives.
I am looking forward with more zest than before to 2021, a year when new data on XP, DNA regeneration, stroke and information on vitiligo will be received, while new products will come to the fore. The Group will expand, and new skills will be added. I thank all shareholders for your support, our staff for their commitment during a difficult year, our prescribing physicians for your incessant dedication to our patients, and the coherent Board of Directors for its enthusiasm and work for the Company.
Stay safe and reflect on all the globe has gone through, 2021 will be better and stay tuned for the News Communiqué I in January.
Philippe Wolgen
Table of Announcements

1SCENESSE® (afamelanotide 16mg) is approved in the European Union as an orphan medicinal product for the prevention of phototoxicity in adult patients with EPP. SCENESSE® is approved in the USA to increase pain free light exposure in adult EPP patients with a history of phototoxicity. Information on the product can be found on CLINUVEL’s website at www.clinuvel.com.
Download Pdf