2021 Announcements Presentation
Investor Presentation – Daiwa Investment Conference Tokyo 2021
10 March 2021 – Melbourne, Australia
On 10 March 2021 CLINUVEL presented at the Daiwa Investment Conference on CLINUVEL’s evolution and strategy. A copy of the presentation with speaker’s notes is available to view and download below.

The first phase was from formation and initial strategy to 2005: CLINUVEL’s core technology, afamelanotide, was invented at the University of Arizona in the late 1980s and acquired by CLINUVEL in 1999. Afamelanotide is a synthetic peptide which mimics the naturally occurring alpha-melanocyte stimulating hormone (α-MSH). The peptide stimulates the production of eumelanin which provides protection from UV and visible light. The period to 2005 sought to apply the technology to develop a tanning preparation, but this more cosmetic than medicinal strategy did not garner support from medical practitioners and regulators. Hence, the Company’s strategy was unsupported and needed to change.The second phase was drug development and commercialisation: In 2005 a new management team, vision and strategy were put in place. From 2005 to 2020 we developed and commercialised a novel drug for an unmet medical need. SCENESSE® (afamelanotide 16mg) was developed as a controlled release subcutaneous injectable implant; erythropoietic protoporphyria (EPP) was selected as the lead indication; we completed clinical studies; obtained regulatory approvals; and commercialised SCENESSE® as the world’s first systemic photoprotective.The European Medicines Agency (EMA) and US Food and Drug Administration (FDA) approved SCENESSE® for adult EPP patients in 2014 and 2019, respectively. Commercial distribution commenced in the European Union in June 2016 and the USA in April 2020. After more than four years of commercial operations, we have built a viable business generating positive cashflow and profit, with a strong balance sheet and cash reserves sufficient to finance planned organic growth.The third, current and most exciting phase of CLINUVEL’s evolution is to expand access to SCENESSE® in EPP and to translate the technology to new targeted indications and healthcare solutions for broader audiences. CLINUVEL is well positioned to grow and diversify, despite the challenging operating environment.
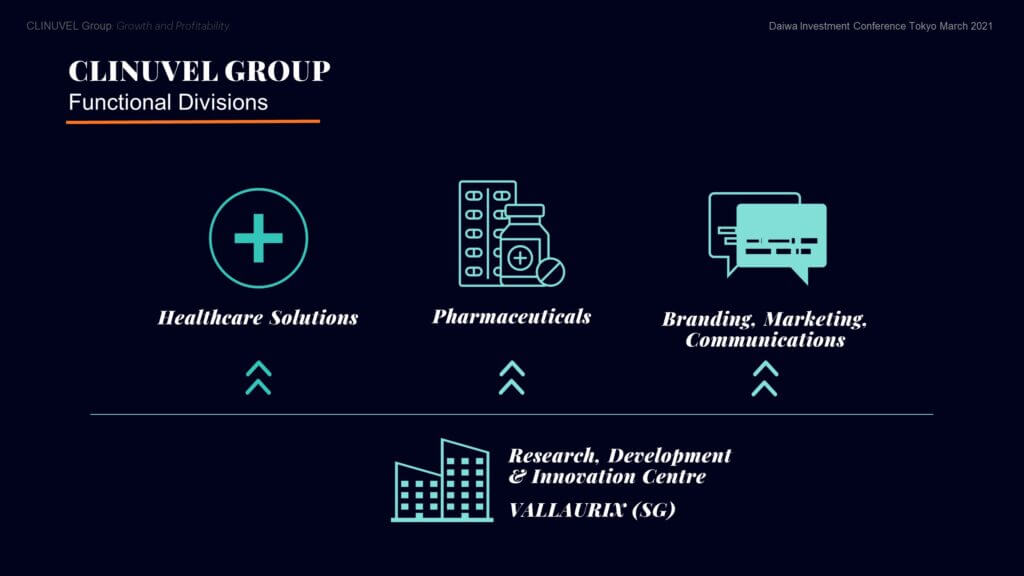
The Pharmaceuticals Division – CLINUVEL’s core business, focussed on developing and delivering drugs for patients with unmet medical need.
The Healthcare Solutions Division – concentrated on non-prescription products derived from the knowhow and active ingredients used in the Pharmaceuticals Division.
The Communications, Branding & Marketing Division which prepares communications to wider differentiated audiences, positioning the Group for broader engagement.
Underlying this divisional structure is the Research, Development & Innovation (RDI) Centre in Singapore, researching molecular science, biology, and follow-on formulations.

Afamelanotide is the active ingredient in SCENESSE®. The drug:
was developed as a controlled release subcutaneous injectable implant formulation, administered in an outpatient setting;
has been shown to reduce the incidence and severity of phototoxic reactions and increase the time EPP patients can expose to light without phototoxicity;
is monitored in post-authorisation use in EPP patients by an extensive pharmacovigilance program; and
has maintained a positive safety profile from over 10,000 doses to over 1,400 individuals worldwide.α-MSH is part of a family of peptides known as melanocortins, all of which are cleaved from the precursor polypeptide proopiomelanocortin (POMC) and bind to specific melanocortin receptors throughout the body. There is growing recognition of their role in the function of key organs of the body.The safety and potential of SCENESSE® to treat other indications is the basis of CLINUVEL’s strategy to translate the technology to new indications.

In the US, we distribute largely through certified dermatologists. We have trained and accredited 34 Specialty Centers compared to 30 planned by the end of 2021. Treatment under Prior Authorization means each patient confirms insurance coverage before treatment by their Specialty Center. Additionally, Centers require confirmation from the insurer of the treatment codes to charge for the medical consultation and drug administration. A Savings Program is operating for US EPP patients working off individual Insurance Plans. The US label allows one implant every two months.
Distribution in Europe is through EPP Expert Centres, trained and accredited by CLINUVEL. Demand for SCENESSE® in Europe has been strong with patient retention of 94 to 97% in the European Economic Area. COVID impacted the treatment of EPP patients in March to May 2020 when a few centres were not able to provide treatment due to priority to COVID patients, and some EPP patients could not travel to get treatment. Since then, notwithstanding the risk of new waves of COVID, treatment has largely normalised in Europe.SCENESSE® was approved by the Australian Therapeutic Goods Administration (TGA) in October 2020 for the prevention of phototoxicity in adult patients with EPP and granted market access in Israel as a first line treatment for the prevention of phototoxicity in adult patients with EPP in February 2021. We are committed to facilitating treatment access to SCENESSE® for EPP patients worldwide.
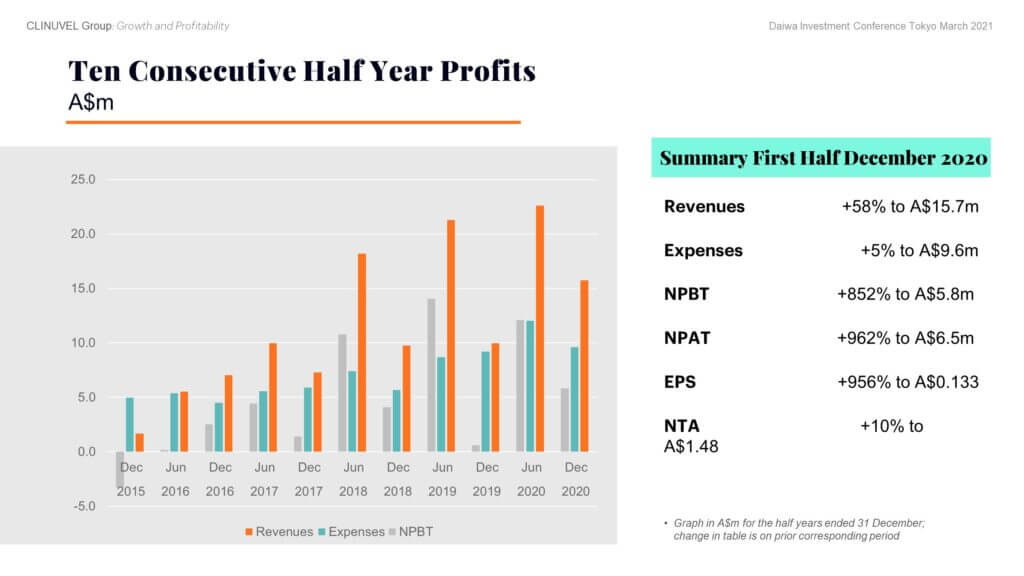
In more recent financial news, cash receipts in the 2020 calendar year achieved a record level of A$33.053 million. CLINUVEL also posted a 58% increase in revenues in the half year to December 2020. Both cash and revenues reflect the normalisation of treatment activity in Europe, (after an initial COVID related impact), and the first contributions from US operations. Growth in expenses was contained to 5% in the half year. The profit before tax of A$5.811 million was the tenth consecutive half year profit and a record for a December half year since the commencement of commercial operations. This demonstrates the resilience of the business and affirms the appropriateness of the Group’s long-term strategy and the efficacy of the business model to distribute direct to the medical practitioners administering the drug to patients.
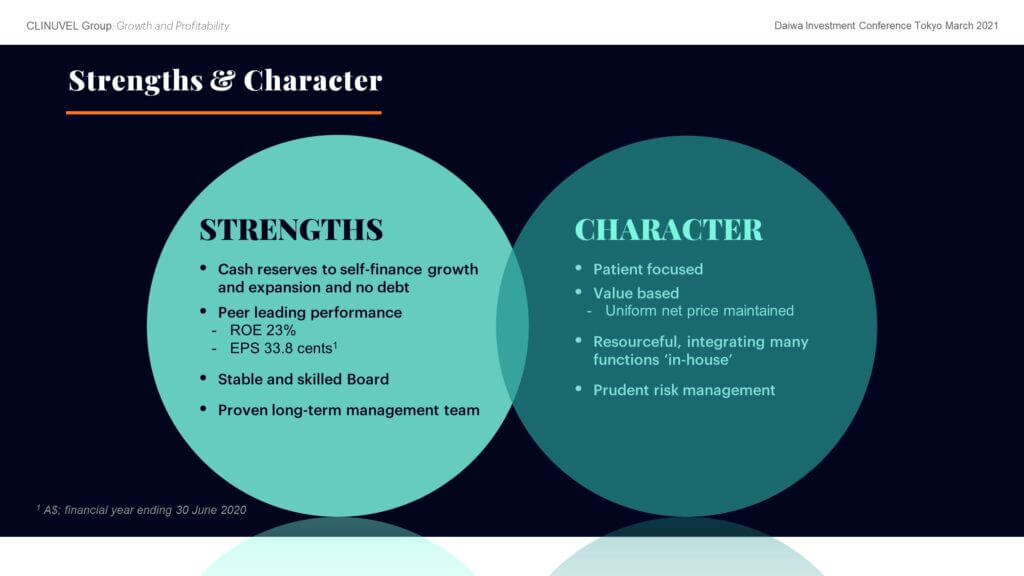
a long-term strategy to develop and commercialise a novel technology for an unmet medical need;
the economic management of expenses to develop SCENESSE® for A$154 million, far less than the US$1-2 billion typically required to develop a new drug;
self-management of the development of SCENESSE®, clinical studies, liaison with regulators and distribution of SCENESSE®; and
the accumulation of cash reserves to manage adversity in all economic conditions.
These strengths and characteristics benefit shareholders by supporting the long-term value of the Company and underpin the business as it implements its forward strategy to grow the commercial operations based on SCENESSE® and diversify into new indications.
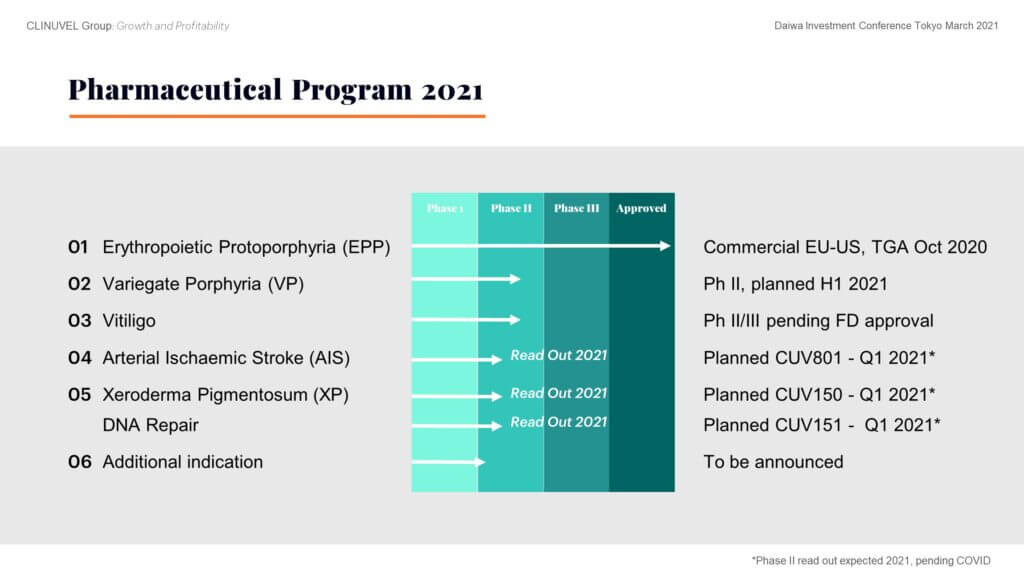
Vitiligo progression depends on agreement on final protocol with the FDA. There is consensus among our scientific team and global vitiligo experts to focus drug availability on patients with darker skin complexions. These darker skin types more prominently exhibit the contrast between pigmented and depigmented skin.The DNA Repair Program continues, following the treatment of the first xeroderma pigmentosum (XP) patient in September 2020. During 2021 we expect readouts from the XP study (Phase II CUV150) and a study in healthy subjects (CUV151). In parallel, we look to a fast outcome from the stroke study (Phase II CUV801), but note, these are all conditional on COVID restrictions being lifted.An additional indication is also to be announced.
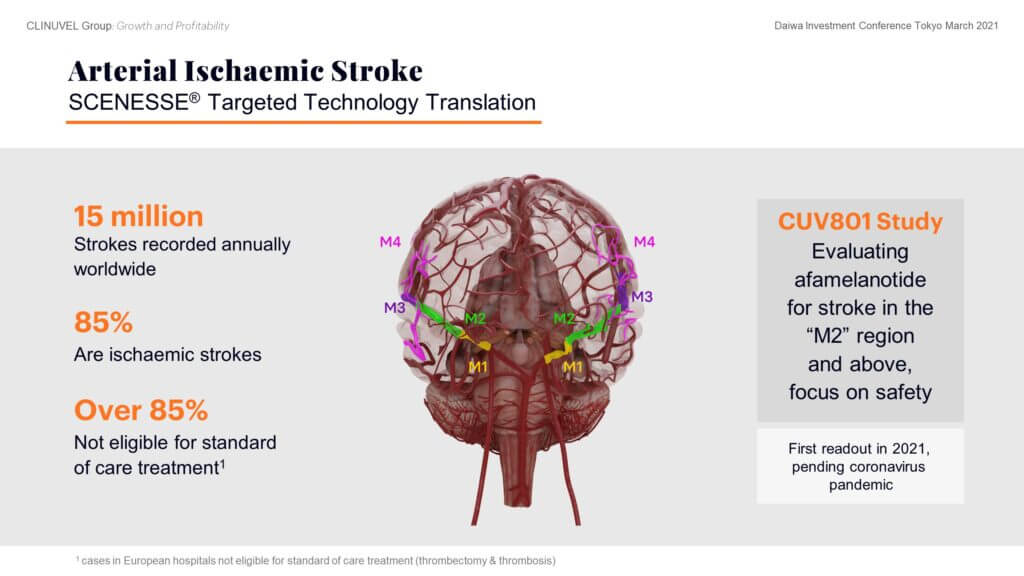
First, the role of afamelanotide in treating arterial ischaemic stroke patients. Tragically, many ischaemic stroke patients either have lasting functional impairment or do not survive the clot that has been formed and dislodged in their brain. We understand afamelanotide may well play a role in treating ischaemic stroke by rapidly exerting its effects to protect brain tissue, acting on blood vessels to optimise blood flow, and reducing the size of swelling in the brain following a stroke. Our clinical focus is on patients with ischaemic strokes in the upper regions of the brain, the so-called “M2” branches and further up in the brain. Of the 15 million strokes reported each year, over 85 percent are ischaemic strokes, and a majority of these are untreatable with the current standard of care, representing a genuine unmet medical need.

Given DNA damage and the risk of skin cancer affects almost all fair-skinned individuals on the planet, the relevance of the DNA Repair Program is clear.

The Strategic Update of October 2020 explained that our melanocortin technology lends itself uniquely to a dual strategy to serve prescription and non-prescription markets. This can be reviewed on the CLINUVEL website (at www.clinuvel.com). A key, but unexpected, part of the pharmaceutical legacy is that the very assurance the Company demonstrated to regulators on the safety of afamelanotide has now become one of CLINUVEL’s unique propositions and the prime asset enabling us to develop derivative products for wider retail markets. Our technology originates from a highly regulated environment and can be translated to non-prescription products (pharmaceumables) with an emphasis on SAFETY and genuine care for human biology. This is a unique position which very few companies would be able to emulate and given the assessed demand for authentic dermatocosmetics, we are well positioned in this underdeveloped market segment.
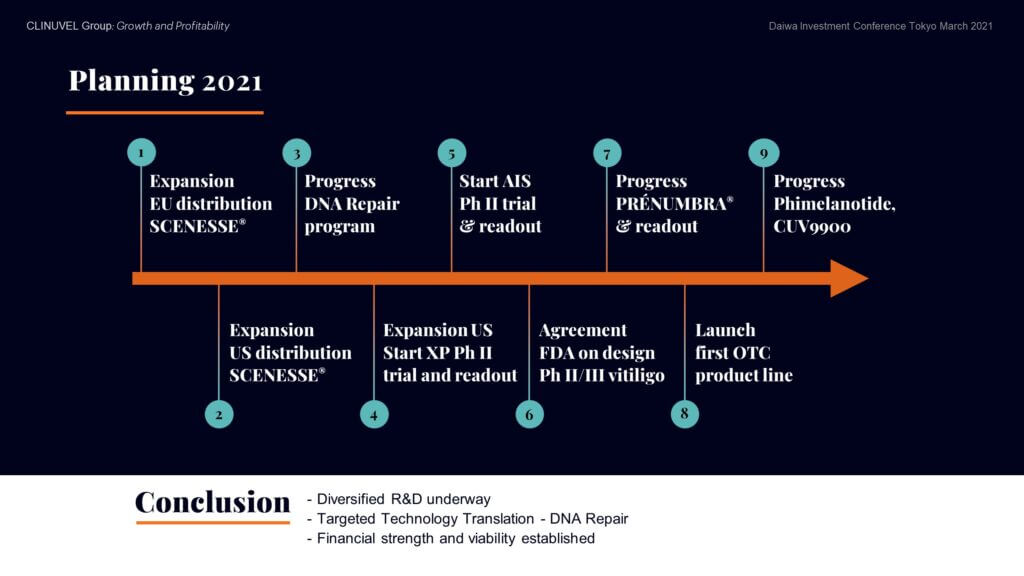
Following an eventful 2020 with new milestones achieved and progressive news flow from the Company on its growth and expansion, nine key objectives listed for 2021 form the basis of ongoing news.
In conclusion, CLINUVEL’s strategy is to become a diversified operation based on the dual progression of the core Pharmaceuticals Division and the new Healthcare Solutions Division. New to shareholders is our unfolding of the ischaemic stroke program and the DNA Repair Program with trials in XP and in healthy volunteers. We are diversifying our R&D and translating our technology from a position of financial strength and viability.

About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL PHARMACEUTICALS LTD (ASX: CUV; NASDAQ INTERNATIONAL DESIGNATION ADR: CLVLY; XETRA-DAX: UR9) is a global and diversified biopharmaceutical company focused on developing and commercialising treatments for patients with genetic, metabolic, and life-threatening disorders, as well as healthcare solutions for the general population. As pioneers in photomedicine and understanding the interaction of light and human biology, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair and acute or life-threatening conditions. These patient groups range in size from 5,000 to 45 million worldwide. CLINUVEL’s lead compound, SCENESSE® (afamelanotide 16mg), was approved by the European Commission in 2014, the US Food and Drug Administration in 2019, and the Australian Therapeutic Goods Administration in 2020 for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). More information on EPP can be found at http://www.epp.care. Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore, and the USA. For more information please go to http://www.clinuvel.com.SCENESSE® and PRÉNUMBRA® are two of several registered trademarks of CLINUVEL PHARMACEUTICALS LTD.
Previous
Next
Download Presentation & Speaker’s Notes