CLINUVEL Delivers Record Revenues and Profit
| Melbourne, Australia, 26 August 2021 | ASX: XETRA-DAX: NASDAQ INTERNATIONAL DESIGNATION: |
CUV UR9 CLVLY |
| An Investor Webinar will be held today (26 August) at 18:00-18:30 AEST (10:00-10:30 CEST) – see details below. | ||
Key Highlights, Year Ending 30 June 2021
| Consolidated Entity | 30 June 2021 | 30 June 2020 restated |
|---|---|---|
| Total Revenues1 | $48,451 | $33,911 |
| Total Expenses1 | $22,738 | $22,367 |
| Net Profit before income tax1 | $25,713 | $11,541 |
| Profit after income tax expense1 | $24,728 | $15,051 |
| Basic Earnings per Share | $0.50 | $0.31 |
| Net Tangible Assets backing per Share | $1.91 | $1.35 |
| Dividend distribution per Share | $0.025 | $0.025 |
| All figures reported in Australian dollars (A$) for the financial years ending 30 June. 1 Figures in A$ ‘000s. Refer to the Appendix 4E Preliminary Final Report released to the Australian Securities Exchange for details | ||
CLINUVEL today announced record annual revenues ($48.451 million) and profit ($25.713 million before tax), released in its Appendix 4E Preliminary Final Report (audited) for year ending 30 June 2021 (FY2021).
“Today’s record financial result, achieved in our fifth year of commercial operations, validates the strength of CLINUVEL’s business model and the strategic direction of the Company,” CLINUVEL’s Chief Financial Officer, Mr Darren Keamy said. “The result has been driven by strong patient demand in Europe and in the USA, despite a challenging operating environment.
“The progress in the US in the first full year of commercial operations is ahead of our planning, with over 40 Specialty Centers trained and accredited to administer SCENESSE® and over 60 national and state private insurers reimbursing EPP patients’ treatment.1 Our US roll out, combined with ongoing demand in Europe, has helped deliver strong growth in revenues with only a relatively modest increase in overall expenses.
“The Company’s cash reserves allow us to declare a dividend today to recognise the loyalty of long-term shareholders, while still being in a position to finance our planned organic expansion, translating our technology to benefit other patients and the general population at higher risk of DNA damage,” Mr Keamy said.
Fourth Consecutive Annual Dividend
The CLINUVEL Board has declared a fourth consecutive annual unfranked dividend of $0.025 per ordinary share following the financial results for the year ending 30 June 2021. The Company has previously issued unfranked dividends for the financial years ending 30 June 2020 and 2019 of $0.025 and 2018 of $0.02, respectively. Subject to the Company maintaining sufficient cash reserves, the key dates for the dividend are:
- Ex-dividend date: 02 September 2021;
- Record date: 03 September 2021; and
- Payment date: 17 September 2021.
Dividends are available to Australian and overseas registered shareholders, including holders of CLINUVEL’s Level 1, American Depository Receipts. Prior to the record date, shareholders are encouraged to confirm their personal shareholder information, including payment election information, with the share registrar.
Long-Term Strategy and Business Model
CLINUVEL has implemented a long-term strategy focussed on the development and commercialisation of its novel drug technology for the genetic metabolic disorder EPP.
The Company has adopted a business model which integrates key functions such as research, development and innovation, regulatory affairs, pharmacovigilance reporting to regulatory authorities and direct distribution to the treatment centres. Reflecting its corporate values of respect and equal opportunity, a uniform net price policy operates within each jurisdiction where commercial operations take place.
Five Years Of Revenue and Profit
The Company has achieved five consecutive years of positive cashflow, revenues and profit, while maintaining control over its operating expenses to facilitate growth.
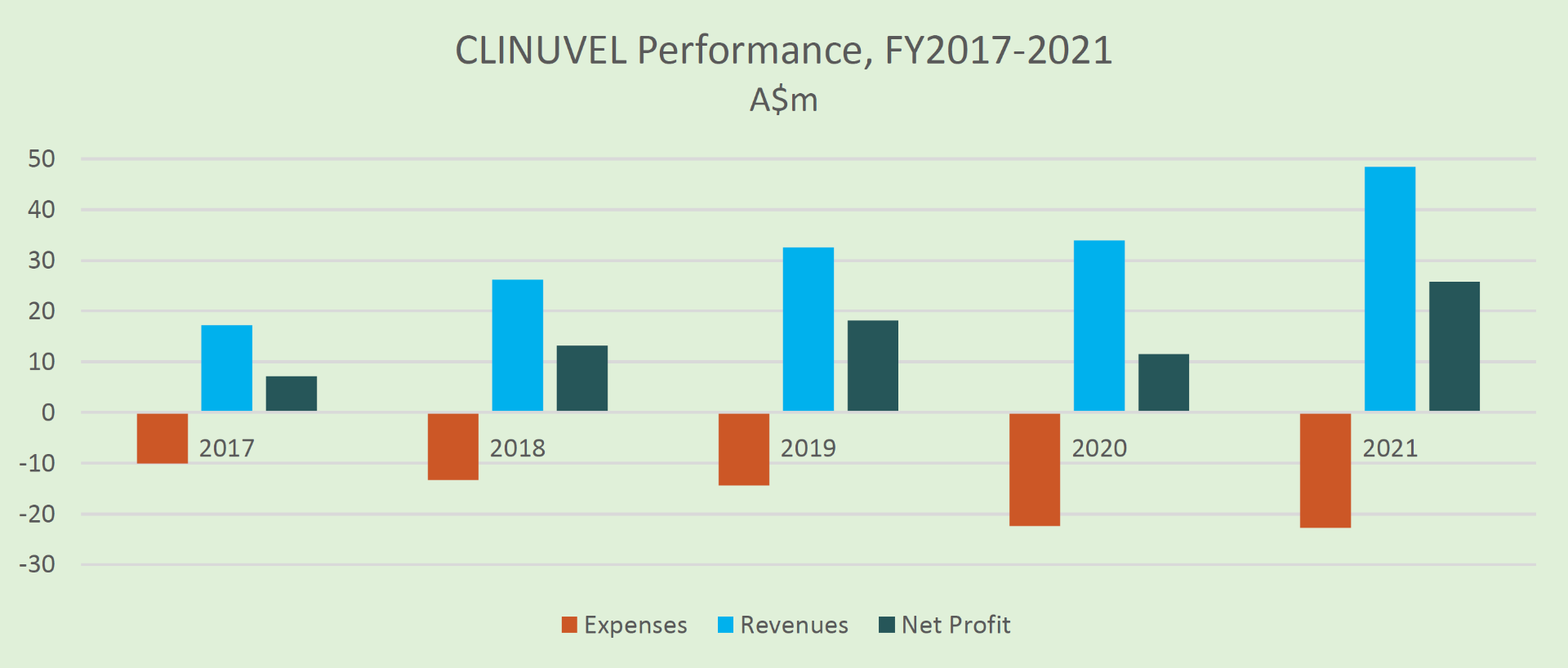
Growth and Expansion Strategy
The Group is committed to growing its commercial operations in Europe, the USA, Israel, and other countries. CLINUVEL’s has expanded its clinical program to evaluate afamelanotide’s ability to assist the repair of ultraviolet-induced DNA damage and improve the lives of patients suffering Arterial Ischaemic Stroke. The Group is also developing non-prescription, dermatocosmetic products for the wider population at high risk of ultraviolet and high energy light insult. This growth strategy is enabled by the Company’s cash reserves which stand at $82.691 million as of 30 June 2021.
The expansion of the Group is supported by its new divisional structure, comprised of the Pharmaceuticals; Healthcare Solutions; Communications, Branding & Marketing; and Manufacturing, underpinned by the Research, Development & Innovation Centre in Singapore.
More information on the Company’s strategy can be found at www.clinuvel.com.
Clinuvel Briefing
CLINUVEL will host an investor and analyst webinar at 18:00 AEST today to review the results of the Financial Year End 2021. Participants can register using the link below:
Investor Zoom Webinar 18:00-18:30 AEST (10:00-10:30 CEST) today (26 August)
To participate, please register using this link:
https://zoom.us/webinar/register/WN_0jevdk07TFaeOAgbsW1ruw
Questions may be tabled as you register or during the webinar
– End –
CLINUVEL’s Appendix 4E is available on the Company’s website, www.clinuvel.com.
SCENESSE® (afamelanotide 16mg) is approved in the European Union and Australia as an orphan medicinal product for the prevention of phototoxicity in adult patients with erythropoietic protoporphyria (EPP). SCENESSE® is approved in the USA to increase “pain-free” light exposure in adult EPP patients with a history of phototoxicity. Information on the product can be found on CLINUVEL’s website at www.clinuvel.com.
About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL PHARMACEUTICALS LTD (ASX: CUV; NASDAQ INTERNATIONAL DESIGNATION ADR: CLVLY; XETRA-DAX: UR9) is a global and diversified biopharmaceutical company focused on developing and commercialising treatments for patients with genetic, metabolic, and life-threatening disorders, as well as healthcare solutions for the general population. As pioneers in photomedicine and understanding the interaction of light and human biology, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair and acute or life-threatening conditions. These patient groups range in size from 5,000 to 45 million worldwide. CLINUVEL’s lead compound, SCENESSE® (afamelanotide 16mg), was approved by the European Commission in 2014, the US Food and Drug Administration in 2019 and the Australian Therapeutic Goods Administration in 2020 for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). More information on EPP can be found at http://www.epp.care. Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore and the USA. For more information please go to http://www.clinuvel.com.
SCENESSE® and PRÉNUMBRA® are registered trademarks of CLINUVEL PHARMACEUTICALS LTD.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
Media Enquiries
Monsoon Communications
Mr Rudi Michelson, 61 411 402 737, rudim@monsoon.com.au
Head of Investor Relations
Mr Malcolm Bull, CLINUVEL PHARMACEUTICALS LTD
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance, or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products, the COVID-19 pandemic affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg); our ability to achieve expected safety and efficacy results through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; any failure to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Preliminary Final Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on the forecasts and estimates is available on request. Past performance is not an indicator of future performance.
www.clinuvel.com
Level 11
535 Bourke Street
Melbourne – Victoria, Australia, 3000
T +61 3 9660 4900
F +61 3 9660 4999