Presentation H.C. Wainwright Global Investment Conference
| Melbourne, Australia, 14 September 2021 | ASX: XETRA-DAX: NASDAQ INTERNATIONAL DESIGNATION: |
CUV UR9 CLVLY |
H. C. Wainwright 23rd Annual Global Investment Conference
13 – 15 September 2021
It is a pleasure to brief you on CLINUVEL today at this prestigious conference This presentation encompasses our evolution from a research and development focused company to a profitable group of companies with growing commercial operations and an active research and development program I will explain our experience and performance to date, and our focus on:
- the growth of commercial operations; and
- the expansion of the research and development pipeline to a range of pharmaceutical and healthcare solutions for patients and specific broader audiences with unmet needs.
Your attention is drawn to our legal notice which highlights that there are many risks that can materialise and impact the achievement of forward looking statements contained in this briefing.
The CLINUVEL Group is a global enterprise, headquartered in Australia with operations in Europe, Singapore, and the USA. Listed on the Australian Securities Exchange in 2001, we also trade, since 2004, on the Xetra-Dax in Germany (as UR9), and the OTC securities market in the USA as a Level One American Depositary Receipt (CLVLY).
CLINUVEL’s initial phase from formation in 1999 to late 2005 was to acquire the core technology, afamelanotide with a view to develop a tanning preparation. A new management team reset the strategy for the second phase of evolution from 2005 to 2020 to develop and commercialise a novel drug for an unmet medical need. SCENESSE® (afamelanotide 16mg) was developed as a controlled release subcutaneous injectable implant; erythropoietic protoporphyria (EPP) was selected as the lead indication; clinical studies were completed; regulatory approvals obtained; and SCENESSE® was commercialised as the world’s first systemic photoprotective. Details of the Company’s developments to date are available on our website, www.clinuvel.com.
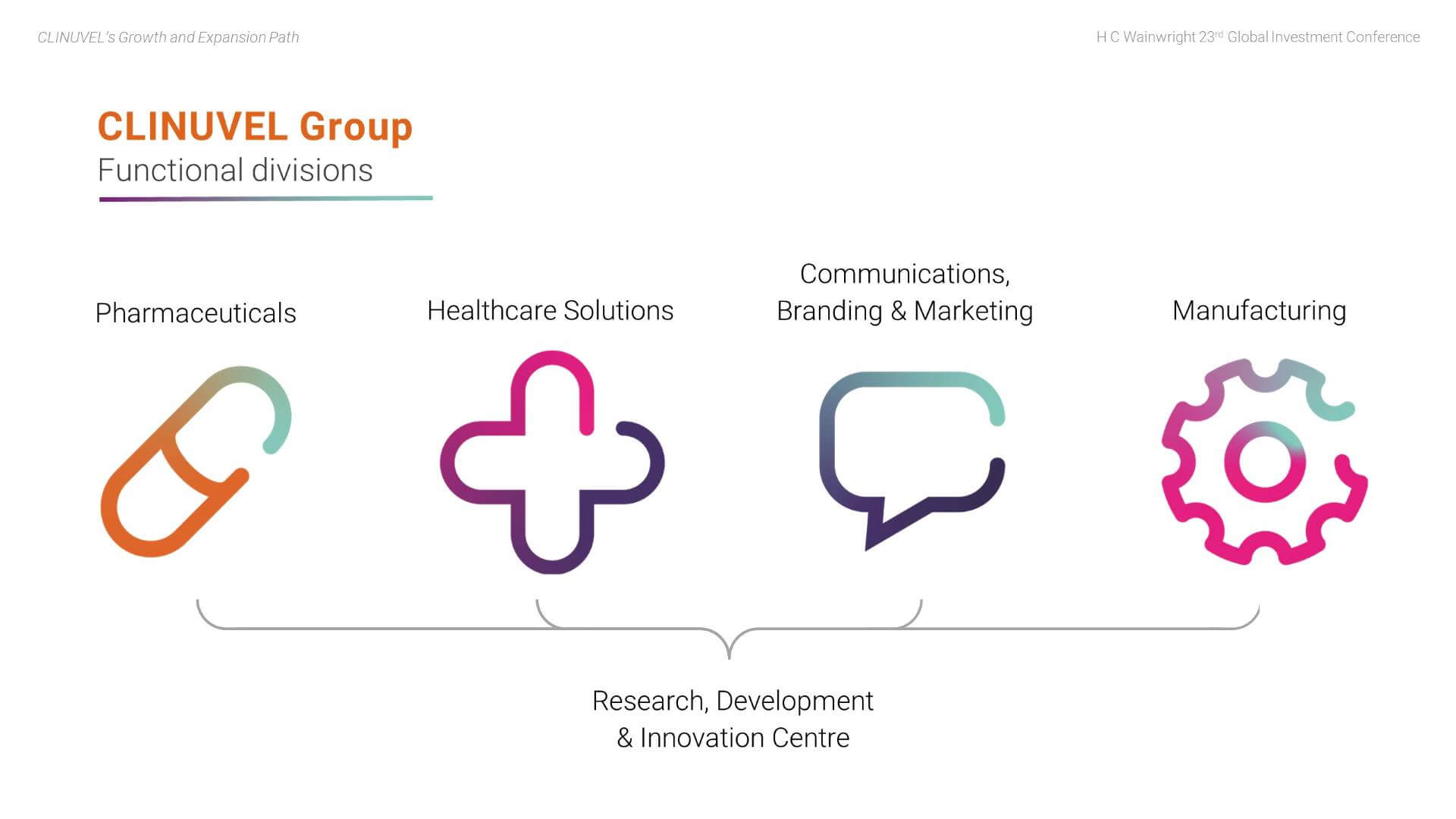
In 2021, we entered the third, current and most exciting phase of CLINUVEL’s evolution to expand access to SCENESSE® in EPP and to translate our technology to new targeted indications and healthcare solutions for broader audiences. To achieve this objective, the Group is organised across four Divisions:
- The Pharmaceuticals Division – CLINUVEL’s core business, focussed on developing and delivering drugs for patients with unmet medical need.
- The Healthcare Solutions Division – concentrated on non-prescription products derived from the knowhow and active ingredients used in the Pharmaceuticals Division.
- The Communications, Branding & Marketing Division – prepares communications to wider differentiated audiences, positioning the Group for broader engagement.
- The Manufacturing Division – focussed on manufacturing novel formulations and products for CLINUVEL and research, development and production for other companies and research groups in the biopharmaceutical sector.
Underlying the divisional structure is the Research, Development & Innovation (RDI) Centre in Singapore, researching molecular science, biology, and follow-on formulations.
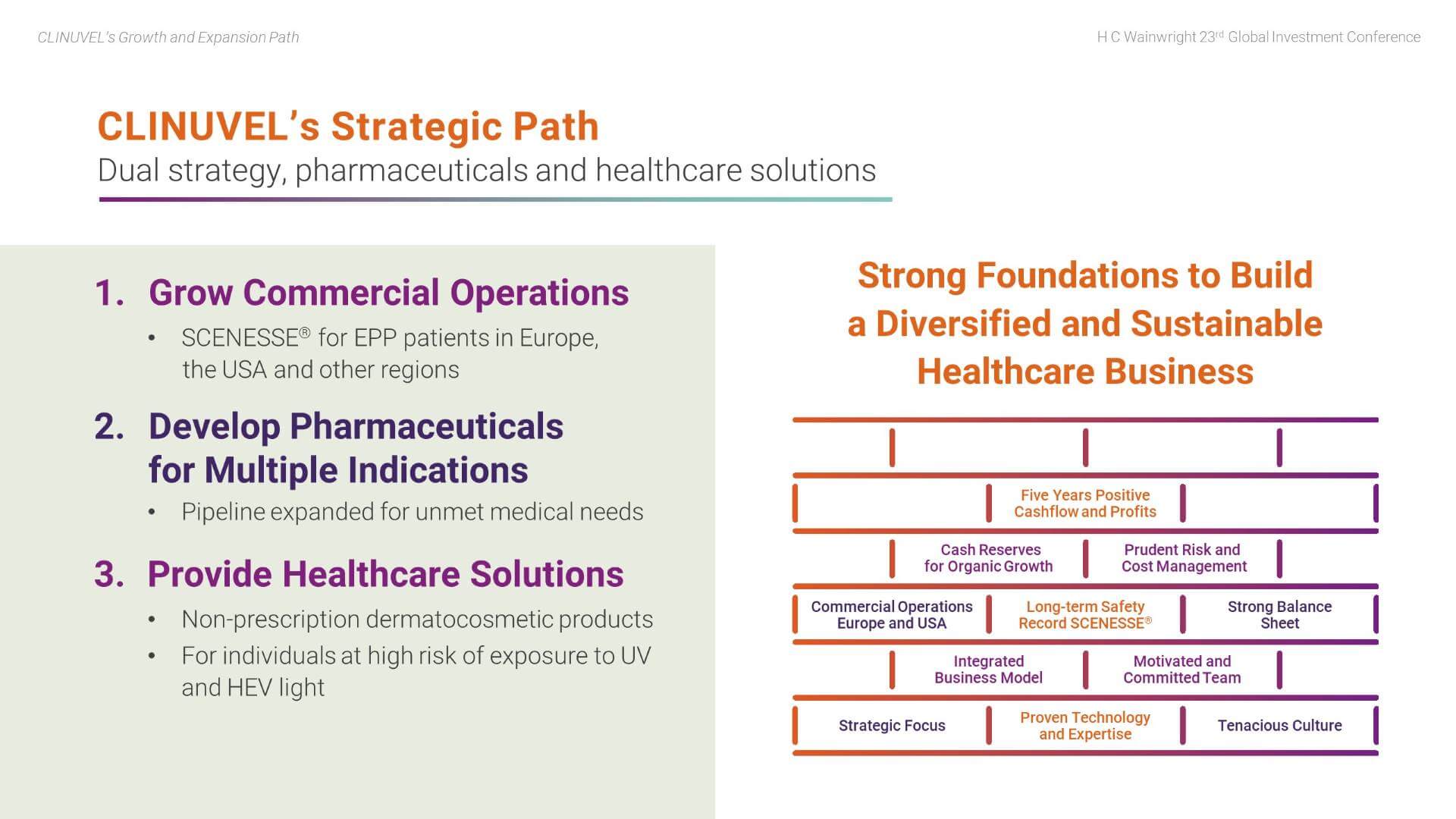
CLINUVEL has established strong foundations to support its planned organic growth and expansion. These foundations include:
- Ongoing commercial operations in Europe and the US which have delivered five years of positive cashflow and profits;
- A strong balance sheet with high liquidity and no debt; cash reserves are sufficient to self-finance planned organic growth;
- Proven technology and expertise; in particular, the long-term safety record of SCENESSE®;
- A motivated and committed team, with a tenacious culture;
- Strategic focus, with prudent risk and cost management; and
- A highly integrated business model.
The Group is pursuing a dual strategy through the Pharmaceuticals Division and the Healthcare Solutions Division to translate its technology to targeted markets. More specifically, we aim to:
- Grow commercial operations based on the pharmaceutical drug, SCENESSE® for EPP patients;
- Develop pharmaceutical products to treat a range of indications with an unmet medical need; and
- Provide non-prescription healthcare solutions to individuals in the wider population at high risk of exposure to ultraviolet (UV) and high-energy visible (HEV) light.
This strategy will build a diversified and sustainable healthcare business and serve to enhance the quality of life and well-being of many patient groups and individuals in the wider population.
Let’s now delve into our proven technology, experience in Europe and the USA, and our financial performance.

The Group’s lead technology is SCENESSE® the only approved treatment for EPP, a poorly characterised, metabolic disorder causing lifelong light intolerance Patients suffer acute phototoxic reactions after exposure to light Without treatment, patients must avoid exposure to light and thus lead a life of social isolation.
The active ingredient of SCENESSE® is afamelanotide a synthetic peptide which mimics the naturally occurring alpha melanocyte stimulating hormone (α MSH) The peptide stimulates the production of eumelanin which provides protection from UV and visible light SCENESSE®:
- was developed as a controlled release subcutaneous injectable implant formulation, administered in an outpatient
- tting;
- has been shown to reduce the incidence and severity of phototoxic reactions and increase the time EPP patients can
- pose to light without phototoxicity;
- is monitored in post authorisation use in EPP patients by an extensive pharmacovigilance program and;
- has maintained a positive safety profile from over 10,000 doses to over 1,400 individuals worldwide.
α-MSH is part of a family of peptides known as melanocortins, all of which are cleaved from the precursor polypeptide proopiomelanocortin ( and bind to specific melanocortin receptors throughout the body There is growing recognition of their role in the function of key organs of the body.
The safety and potential of SCENESSE® and other melanocortins to address other unmet medical and healthcare needs is the basis of CLINUVEL’s strategy to translate the technology to new indications.
We first distributed SCENESSE® for EPP in Italy in 2010 and Switzerland in 2012 under special access programs. Regulatory approval to distribute SCENESSE® in the European Union was granted by the European Medicines Agency (EMA) in 2014 and in the United States (US) by the US Food and Drug Administration (FDA) in 2019. First supply under the EMA approval followed in June 2016 and under the FDA approval in April 2020.
Distribution in Europe is through EPP Expert Centers, trained and accredited by CLINUVEL. Demand for SCENESSE® in Europe has been strong, with patient retention of 94 to 97% in the European Economic Area. COVID-19 impacted the treatment of EPP patients in March to May 2020 when a few Centers were not able to provide treatment due to priority to COVID-19 patients, and some EPP patients could not travel to get treatment. Since then, notwithstanding new waves of COVID-19 and associated restrictions, treatment has largely normalised in Europe.
In the US, we distribute largely through certified dermatologists. We have trained and accredited over 40 Specialty Centers, compared to 30 planned by the end of 2021. Over 60 national and state insurers are reimbursing the cost of treatment, under Prior Authorization. This means each patient confirms insurance coverage before treatment by their Specialty Center. Additionally, Centers require confirmation from the insurer of the treatment codes to charge for the medical consultation and drug administration. A Savings Program is operating for US EPP patients working off individual Insurance Plans. The US label allows one implant every two months.
SCENESSE® was approved by the Australian Therapeutic Goods Administration (TGA) in October 2020 and granted market access in Israel in February 2021 for the prevention of phototoxicity in adult patients with EPP. We are committed to facilitating treatment access to SCENESSE® for EPP patients worldwide.
Reference: Wensink, D., Wagenmakers, M. A. E. M., Barman-Aksözen, J., Friesema, E. C. H., Wilson, J. H. P., van Rosmalen, J., & Langendonk, J. G. (2020). Association of Afamelanotide With Improved Outcomes in Patients With Erythropoietic Protoporphyria in Clinical Practice. JAMA Dermatology, 156(5), 570–575. https://doi.org/10.1001/jamadermatol.2020.0352
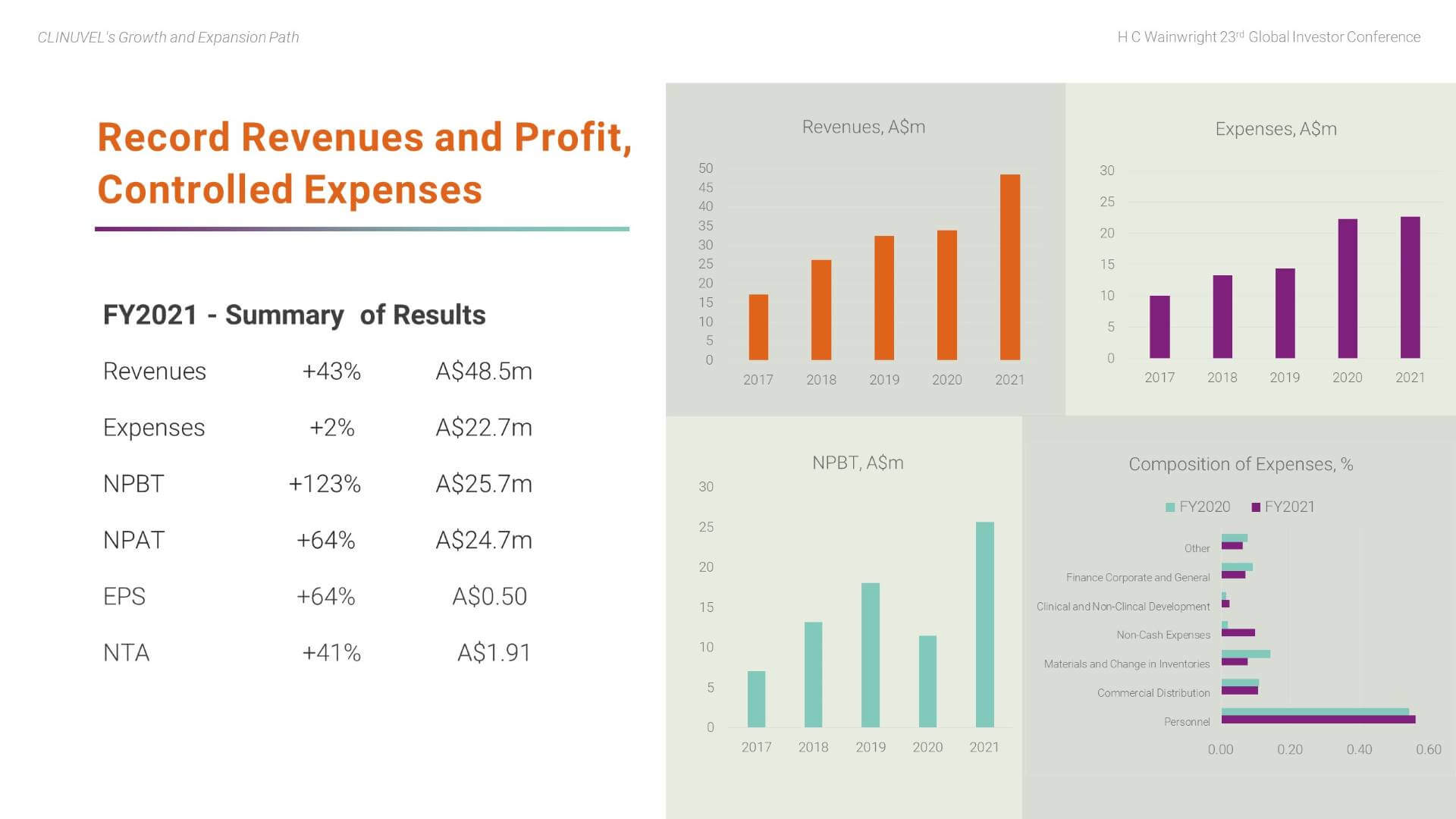
After more than a decade of research and development, CLINUVEL commenced commercial operations in June 2016 and has achieved viability through strong revenue growth and prudent management of expenditures. The first profit was recorded in 2016/17, the first full year of commercial operations. Through the prevailing adversity of the COVID-19 pandemic, CLINUVEL has remained focussed on its long-term strategy and posted a fifth consecutive profit in FY2021.
Revenues rose by 43% to a record A$48.5 million due to the near-normalisation of patient demand in Europe and strong demand for treatment in the USA. In FY2021, we experienced growth in treatment centres; patients; and prescriptions of SCENESSE®. Despite cost pressures on inputs to the business, total expenses were well contained in FY2021 with a 2% increase to A$22.7m; this follows a deliberate and controlled increase of 56% in FY2020 to support the expansion of the Group’s activities. Increases in costs occurred across the business, particularly materials expenses and freight and handling expenses, partly offset by savings in several areas, such as: the cost of responding to regulatory audits; bringing communication and marketing services ‘in-house’; and reduced local and international staff travel due to the COVID-19 pandemic.
Personnel related expenses account for more than half of total expenses, reflecting CLINUVEL’s self-reliant business model in which a range of functions are completed in-house. The Company’s research and development expenses are spread across expense categories of the business. As a guide, total research and development expenses account for around 30% of total annual expenses. This can vary year to year and reflects the essential role of research and development to support the planned expansion of medicinal and dermatocosmetic solutions to new patient groups and individuals in need.
The record profit was A$25.7m before tax and A$24.7m after tax. These results reflect the disciplined implementation of CLINUVEL’s long-term, focussed strategy, and the efficacy of a highly integrated business model. The resilience and sustainability of the business, particularly during the adverse global economic impact of the COVID-19 pandemic over the last 18 months, is demonstrated. The Company has a track record of positive annual cashflow and profitability. It has built cash reserves sufficient to self-finance planned organic growth and declared dividends for the last four financial years (A$0.025 in FY2021, FY2020 and FY2019 and A$0.02 in FY2018). This solid financial foundation supports the expansion of the Company’s research and development program into treatments for other indications to assist patient groups with unmet medical needs and to provide healthcare solutions to individuals who are at high risk from exposure to UV and HEV light.
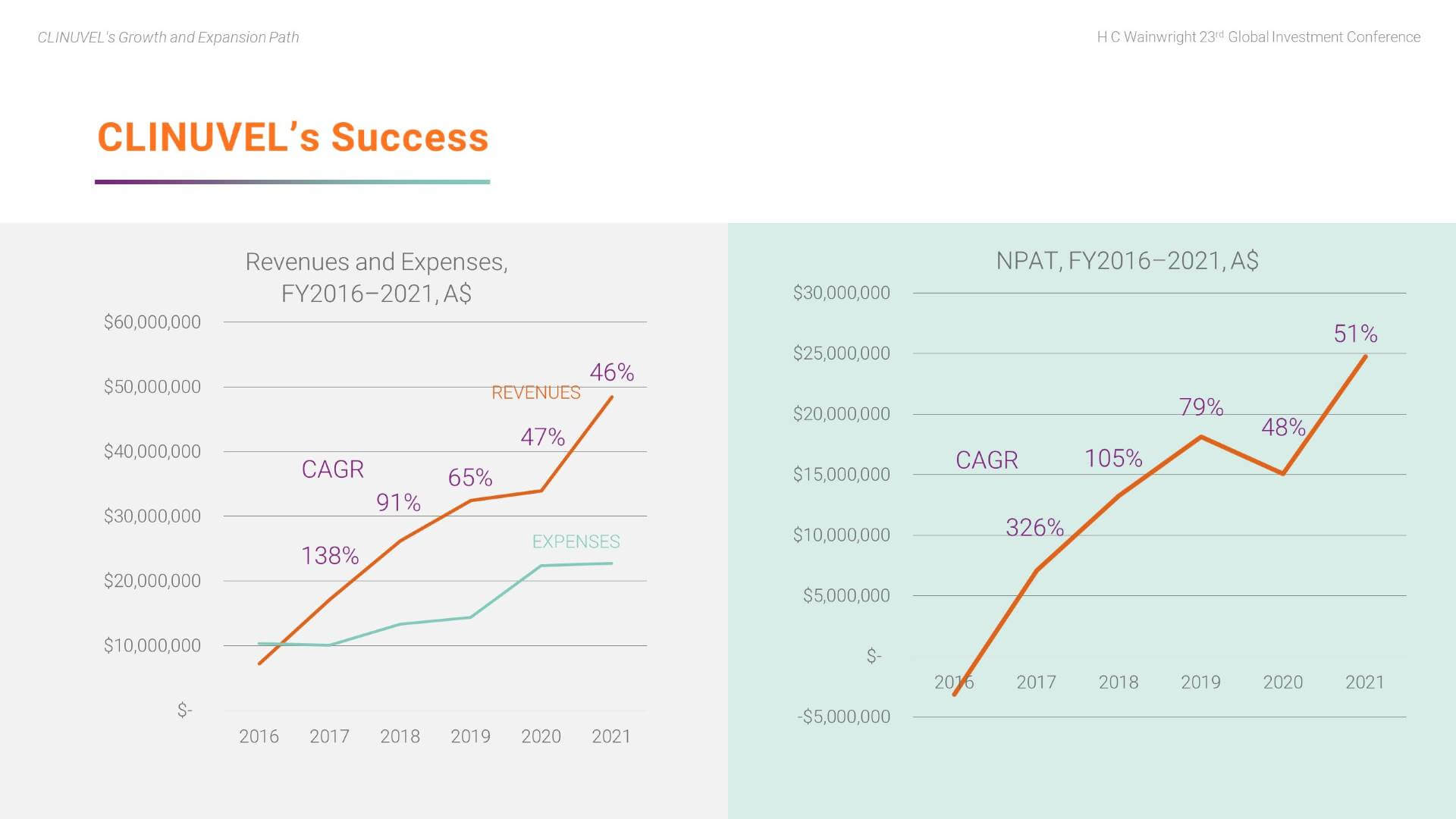
Here we highlight key aspects of CLINUVEL’s success to date.
We can see the strong annual increase in revenues relative to the controlled growth in expenses over the five years of commercial operations. The CAGR of revenues is clearly strong.
We have also achieved a strong CAGR in net profit.
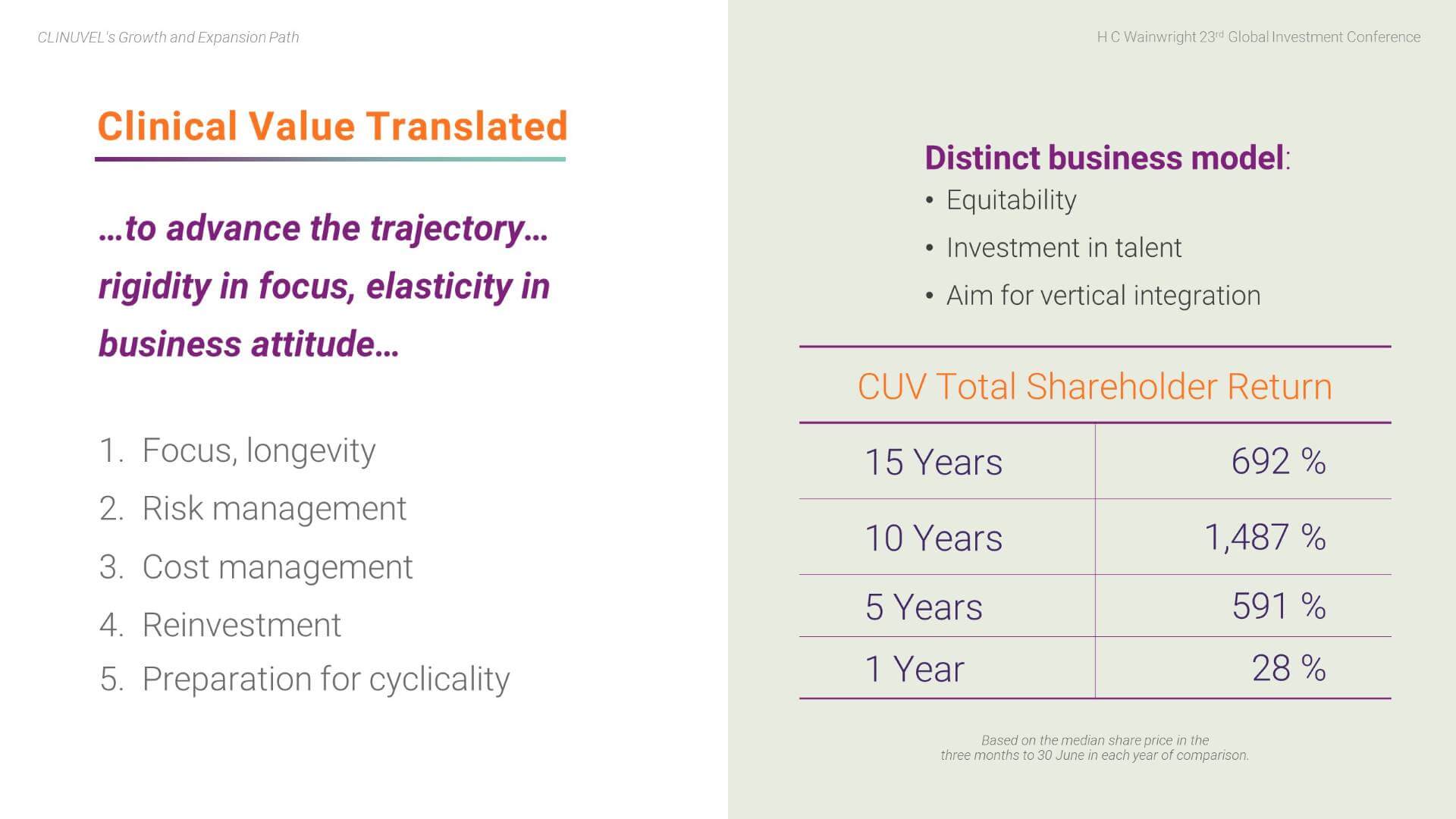
The translation of clinical value has been enabled by a distinct business model, particularly the equitable treatment of patients, centres, and payors, investment in the development of people, and integration of key functions of the business ’in-house’, as well as:
- Focus and longevity of management and the Board;
- Prudent risk and cost management;
- Constant reinvestment in the business; and
- Preparation to manage economic cycles.
In terms of total shareholder return, the change in the share price over the past year, 5 years, 10 years and 15 years, has been commendable. (This is calculated based on the change in the median three-month price to 30 June for each of the years, as this provides a more meaningful series than comparison of changes on a specific date). When you also note that CLINUVEL has declared a dividend in each of the last four years, (from FY2018 to FY2021), the returns to shareholders are demonstrated.
I’ll now switch focus to our pharmaceuticals and healthcare solutions programs.
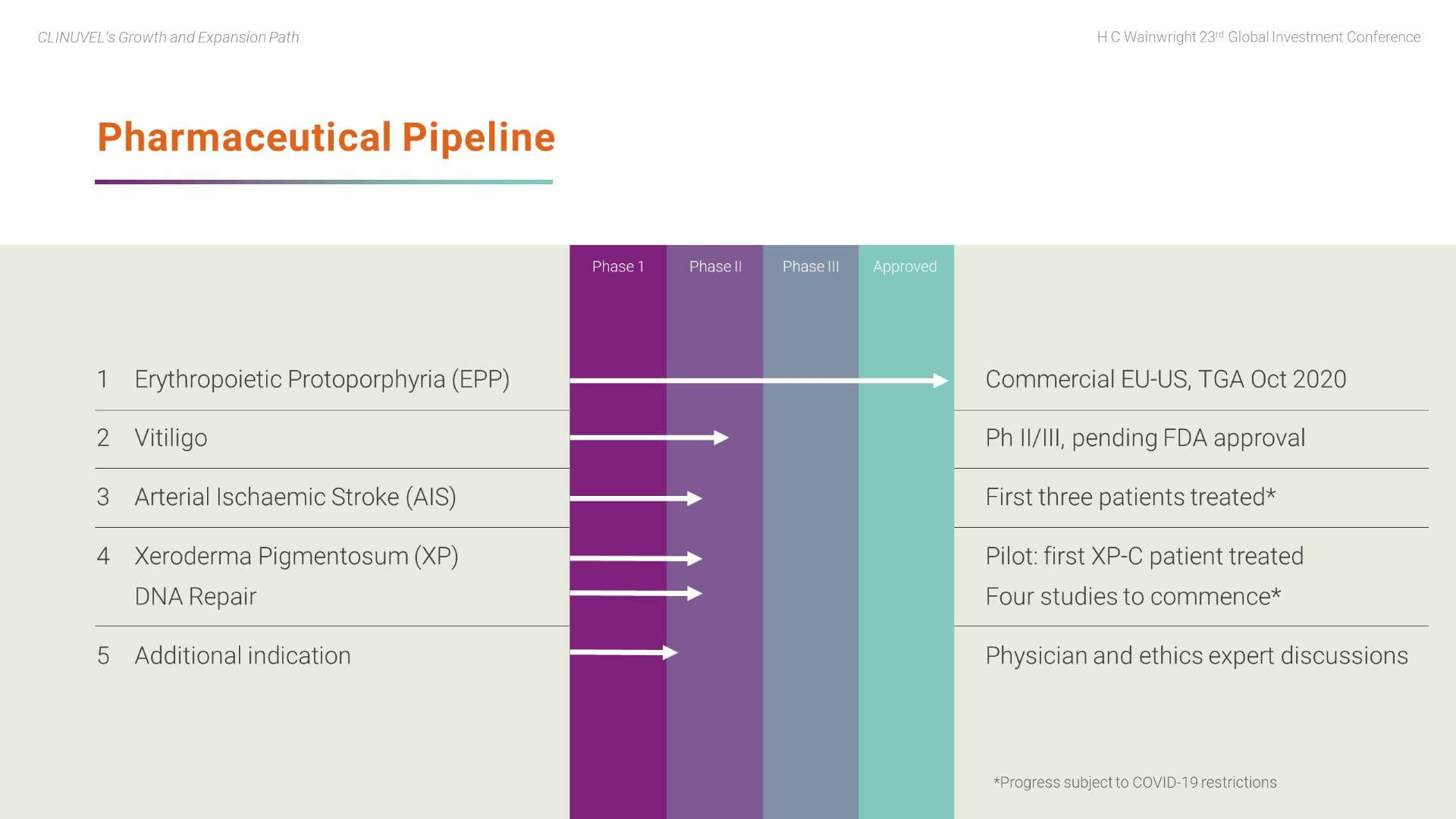
In the core Pharmaceuticals Division, we are working towards a portfolio of prescription products and are currently targeting identified patient populations with afamelanotide. These patient groups lack therapeutic alternatives. The Company decided that future earnings and value should come from its R&D program, thus the focus is to expand from within and utilise our expertise of the pharmacology of melanocortins.
Vitiligo progression depends on agreement on a final protocol with the US Food and Drug Administration (FDA). There is consensus among our scientific team and global vitiligo experts to focus drug availability on patients with darker skin complexions. These darker skin types more prominently exhibit the contrast between pigmented and depigmented skin.
The DNA Repair Program continues, following the treatment of the first xeroderma pigmentosum (XP) patient in September 2020 with four studies set to commence.
In parallel, we recently announced the treatment of three (out of a planned six) patients in the stroke study (Phase II CUV801) and will report read outs when available.
Plans are also progressing to develop a further clinical indication.
In this and the next slide, we highlight two of the clinical programs evaluating novel uses for afamelanotide.
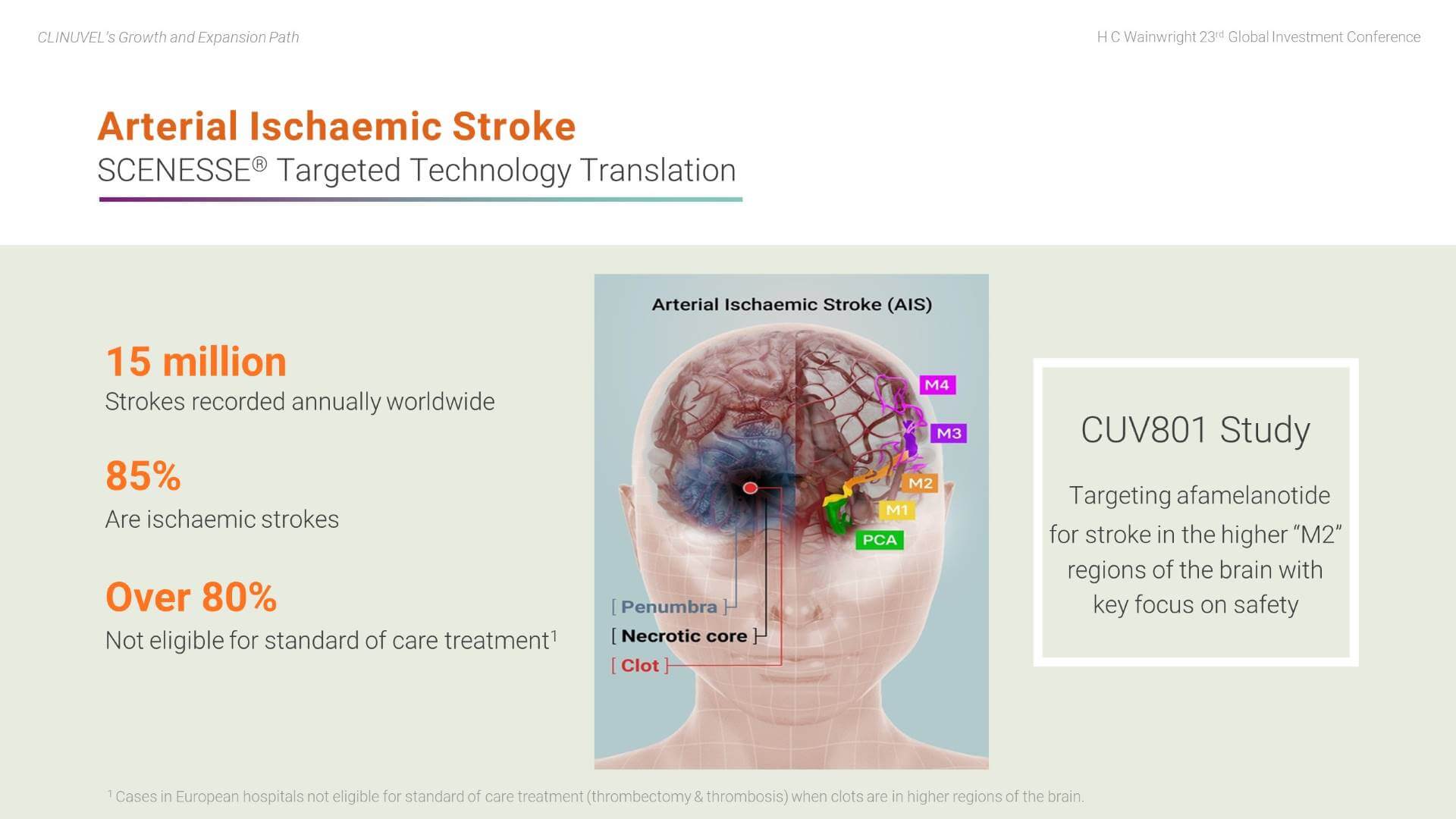
First, the potential for afamelanotide to treat arterial ischaemic stroke (AIS) patients. Tragically, many AIS patients either have lasting functional impairment or do not survive the clot that has been formed and dislodged in their brain. We understand afamelanotide may well play a role in treating ischaemic stroke by rapidly exerting its effects to protect brain tissue, acting on blood vessels to optimise blood flow, and reducing the size of swelling in the brain following a stroke. Our clinical focus is on patients with clots in the upper regions of the brain, the so-called “M2” branches of the middle cerebral artery and further up in the brain. Of the 15 million strokes reported each year, over 85 percent are ischaemic strokes, and a majority of these are untreatable with the current standard of care, representing a genuine unmet medical need.
The first AIS patient was treated in a world first clinical study in Melbourne in June 2021. During August 2021, we reported that three patients had tolerated afamelanotide well with no treatment related adverse events; two patients significantly improved, one showed no improvement.
The DNA Repair Program is significant because over 2 billion individuals have inefficient DNA repair mechanisms. This makes them susceptible to skin cancer. Afamelanotide is understood to assist the body to repair DNA damaged by exposure of the skin to ultraviolet light. CLINUVEL is now working to prove this in clinical trials.
The clinical focus of the DNA Repair Program is on patients with the rare genetic disorder xeroderma pigmentosum (XP) and healthy volunteers. Last year the first XP patient, with the XP-C variation, received and tolerated the treatment well. In March 2021, we expanded the program to patients with the XP-V variant. The studies are set to commence with the objectives to evaluate afamelanotide in XP-C and XP-V patients in relation to safety, the effect on the integrity of the skin, photoproducts, DNA repair and as a photoprotective drug. They involve the administration of the drug over four months, the taking of skin biopsies to assess UV damage and the administration of ultraviolent radiation to assess erythemal exposure.
The Healthcare Solutions Program will result in the release of topical products in a presently underdeveloped segment of the dermatocosmetics market. Many products promise regeneration and rejuvenation of the skin, but seldom are they based on a new class of molecules tested in human pathology over decades.
CLINUVEL’s focus is to introduce leave-on products, topical formulations based on melanocortin molecules from the Pharmaceuticals Division to provide photoprotection and DNA-restoration for those at high risk of long-term solar and high energy visible (HEV) light insult. These individuals have skin types highly sensitive to light/UV, experience extensive exposure to light due to their work or lifestyle activities, or have received organ transplants. The first product line offers polychromatic protection for extreme conditions; the second product line aims to provide DNA protection and repair.
It is important to recognise that we are not disrupting an existing market, rather introducing new technology, originating from a long executed pharmaceutical program. This specific origin, scientific focus, and pharmacology itself sets CLINUVEL apart from any of the established cosmetic houses.
In summary, CLINUVEL’s strategy is to become a diversified and sustainable healthcare business based on the dual progression of the core Pharmaceuticals Division and the Healthcare Solutions Division. We are diversifying our R&D and translating our technology from a position of financial strength and viability, providing sound returns to shareholders. We will continue to provide regular news and updates on the Company’s progress. You can also expect our ongoing focus and prudence in the execution of our initiatives.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL PHARMACEUTICALS LTD (ASX: CUV; NASDAQ INTERNATIONAL DESIGNATION ADR: CLVLY; XETRA-DAX: UR9) is a global and diversified healthcare company focused on delivering innovative solutions for patients with genetic, metabolic, and life-threatening disorders, as well as lifelong care products for the general population. As pioneers in photomedicine, understanding the interaction of light and human biology, and melanocortin drug development, CLINUVEL’s work has delivered a world-first innovative treatment for patient populations with a clinical need for systemic photoprotection. CLINUVEL’s pharmaceutical R&D programs are focused on melanocortins for use in genetic and metabolic disorders, DNA repair, and acute and life-threatening conditions. These patient groups lack therapeutic alternatives. CLINUVEL’s Healthcare Solutions Division is dedicated to translating the Company’s technology to deliver lifelong care products that protect and repair the skin of those at greatest risk of environmental damage. Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore, and the USA. For more information, please go to www.clinuvel.com.
SCENESSE® and PRÉNUMBRA® are two of several registered trademarks of CLINUVEL PHARMACEUTICALS LTD.