CLINUVEL Reports Record December Half Year Operating Profit
| Melbourne, Australia, 23 February 2022 | ASX: XETRA-DAX: ADR Level 1: |
CUV UR9 CLVLY |
CLINUVEL PHARMACEUTICALS LTD today announced a record December half year operating profit with the release of its Half Yearly Report for the six months ending 31 December 2021.
| Key Highlights, Half Year Ended 31 December 2021 | ||
|---|---|---|
| Consolidated Entity | Result | Changes from same period 2020 |
| Total Revenues | $24,631,000 | +56% |
| Total Expenses | $16,230,000 | +67% |
| Net Profit before income tax | $8,726,000 | +50% |
| Cash and Cash Equivalents | $98,992,000 | 20%1 |
| Net Tangible Asset backing per share | $2.15 | +45% |
|
All figures reported in Australian dollars. Refer to the Appendix 4D Half Year Report released to the Australian Securities Exchange for details. |
||
Record Revenues Drive Profit Outcome
The Company also achieved record revenues for a December half year, and the twelfth consecutive half year profit before tax since commencing commercial operations in June 2016.
“The success of the Company’s deliberate long-term strategy to establish SCENESSE® (afamelanotide 16mg)1 as the global standard of care for erythropoietic protoporphyria (EPP) patients is being reflected in the strength of the Company’s financials and our ability to reinvest for future growth,” CLINUVEL’s Chief Financial Officer, Mr Darren Keamy said.
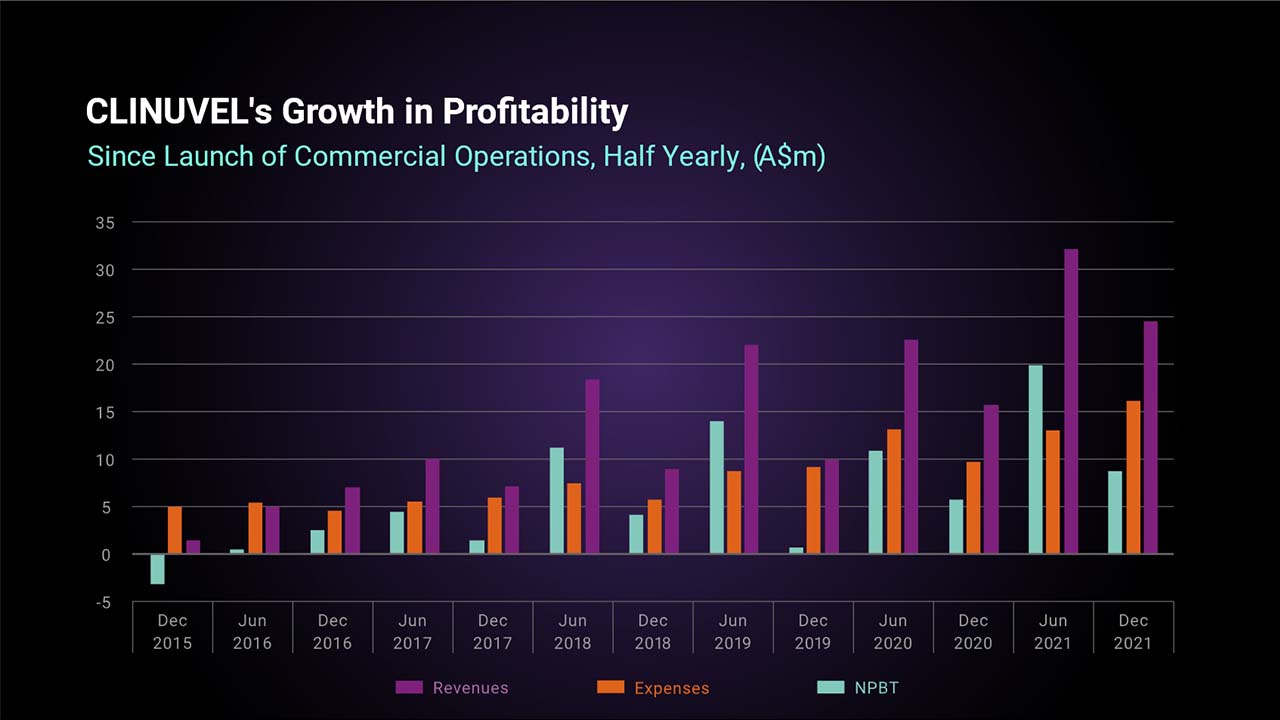
The before tax profit result achieved in the December 2021 half year was 50% higher than the prior corresponding period. The result reflects the firmer demand experienced for SCENESSE® in Europe and consistent growth in the treatment of patients in the United States. Patient treatment by European Expert Centres expanded and rising patient treatment in the US is being facilitated by a larger network of Specialty Centers than originally anticipated, as well as acceptance by over 100 US insurers to reimburse the drug under Prior Authorization arrangements. This has facilitated greater year-round access to treatment for US patients.
The profit after tax result for the period of $5.870 mllion includes a $2.855 million income tax expense and reflects the utilisation of tax losses already brought to account on the balance sheet as a deferred tax asset. This contrasts to the prior reporting period where an income tax benefit of $0.676 million was recorded to recognise prior period tax losses.
Cash and cash equivalents grew by 20% over the six months to 31 December 2021 due to the positive net cash generated from its commercial operations.
Expenses Rise to Support Growth and Expansion
CLINUVEL has established a long track record of prudent cost control, managing expenses to support the Company’s growth and expansion strategy. In line with our business planning, the latest reporting period saw a significant rise in expenses of 67% compared to the prior corresponding period. The largest increases in expenses were in materials and related expenses to ensure inventory requirements can meet clinical demand, followed by personnel related expenses, to build and retain and a team in support of the Company’s strategy.
“The Company is continuing to manage its strong cash position to allow organic growth and retain a financial buffer to withstand unforeseen events like COVID-19 and adverse changes in global economies,” Mr Keamy said.
“We are on track to build a group relying on financial strength and specific expertise in the family of melanocortin hormones. Afamelanotide is only the first offspring to give CLINUVEL a solid foundation for expanding into a diversified and sustainable specialty pharmaceutical.”
“CLINUVEL’s financial management is just one of the pillars providing shareholders stability long-term,” Mr Keamy concluded.
– End –
CLINUVEL’s Appendix 4D is available on the Company’s website, www.clinuvel.com.
CLINUVEL will host an Operations Update Webinar at 18:00 AEDT/08:00 CET. To register, please go to:
https://zoom.us/webinar/register/WN_6yaWw0shRT2PB6Ysq2P7gQ
1 SCENESSE® (afamelanotide 16mg) is approved in the European Union and Australia as an orphan medicinal product for the prevention of phototoxicity in adult patients with erythropoietic protoporphyria (EPP). SCENESSE® is approved in the USA to increase “pain-free” light exposure in adult EPP patients with a history of phototoxicity. Information on the product can be found on CLINUVEL’s website at www.clinuvel.com.
About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL (ASX: CUV; ADR LEVEL 1: CLVLY; XETRA-DAX: UR9) is a global specialty pharmaceutical group focused on developing and commercialising treatments for patients with genetic, metabolic, systemic, and life-threatening, acute disorders, as well as healthcare solutions for the general population. As pioneers in photomedicine and the family of melanocortin peptides, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair, repigmentation and acute or life-threatening conditions who lack alternatives.
CLINUVEL’s lead therapy, SCENESSE® (afamelanotide 16mg), is approved for commercial distribution in Europe, the USA, Israel and Australia as the world’s first systemic photoprotective drug for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore and the USA. For more information, please go to https://www.clinuvel.com.
SCENESSE®, PRÉNUMBRA®, and NEURACTHEL® are registered trademarks of CLINUVEL.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
Media Enquiries
Monsoon Communications
Mr Rudi Michelson, 61 411 402 737, rudim@monsoon.com.au
Head of Investor Relations
Mr Malcolm Bull, CLINUVEL PHARMACEUTICALS LTD
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products; the COVID-19 pandemic and/or other world, regional or national events affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg), PRÉNUMBRA® or NEURACTHEL®; our ability to achieve expected safety and efficacy results in a timely manner through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, Israel, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE®, PRÉNUMBRA® or NEURACTHEL® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology and consumer based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; our ability to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on preliminary and uncertain forecasts and estimates is available on request, whereby it is stated that past performance is not an indicator of future performance.
www.clinuvel.com
Level 11, 535 Bourke Street, Melbourne, Victoria, Australia, 3000, T +61 3 9660 4900, F +61 3 9660 4909