DNA Repair Communiqué I
| Melbourne, Australia, 15 February 2022 | ASX: XETRA-DAX: Level 1 ADR: |
CUV UR9 CLVLY |
Fundamentals of the Cell
Introduction
All human life originates from one single cell, the fertilised egg cell, before rapidly expanding into trillions of specialised cells combined to form complex organ systems over a period of nine short months. To maintain the delicate balance necessary for both survival and diversity, evolution has equipped us with the tools to either remove or tolerate DNA damage. In general, these tools, called DNA repair mechanisms or pathways, curate the DNA molecule to avoid the deleterious consequences of DNA lesions. The importance of these highly specialised pathways is most dramatically demonstrated in the disorder xeroderma pigmentosum (XP), where patients are unable to accurately and completely repair DNA damage. This deficiency ultimately leads to skin cancer and, tragically, a shortened life expectancy for XP patients.
In this DNA Repair Communiqué series, we will explore the world of the cell and provide key insights into the significance of DNA repair. This first Communiqué forms an introductory foundation, outlining fundamental aspects of cell biology and the significance of DNA repair to human survival. With this shared understanding, subsequent Communiqués will explore more detailed topics, including the role of DNA repair in oncogenesis and ageing.
The Fundamentals of Cell Division
The most basic function of the cell cycle is to duplicate with fidelity the vast amount of DNA into two genetically identical daughter cells. This process is divided into two overlapping, phases: nuclear division (mitosis) and the division of the cytoplasm to form two daughter cells (cytokinesis).
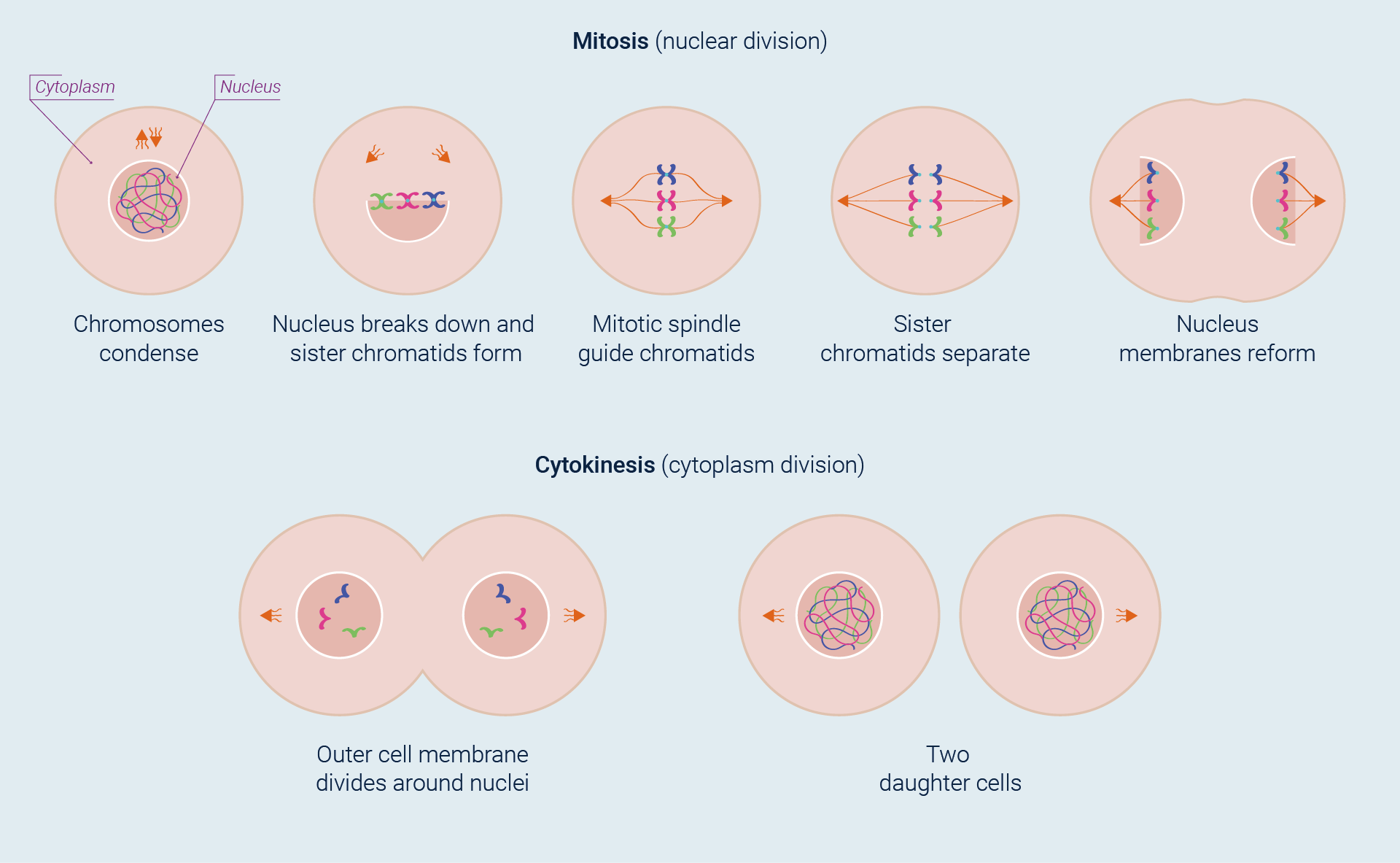

The stars of this mitotic drama are chromosomes. The beginning of mitosis is marked by condensation (coiling and folding) of the cell’s chromatin, generating chromosomes thick enough to be discernible under a microscope. Because DNA replication has already taken place, each chromosome actually consists of two copies that remain attached to each other until the cell divides. If they remain attached, the two new chromosomes are referred to as sister chromatids. As the chromatids become visible, the nuclear envelope breaks into fragments. Then, in a process guided by the microtubules of the mitotic spindle, the sister chromatids separate and, with each now a fully-fledged chromosome, move to opposite ends of the cell. By this time, cytokinesis has usually begun, and new nuclear membranes envelop the two sets of daughter chromosomes as cell division is completed.
The Fundamentals of DNA Repair
In addition to a need for accurate DNA replication, the faithful transmission of genetic information from one generation of cells to the next requires repair of DNA alterations that arise, both spontaneously and from exposure to DNA-damaging environmental agents.
Sunlight, for example, is a strong source of ultraviolet radiation (UVR), which alters DNA by triggering pyrimidine dimer formation—that is, the formation of covalent bonds between adjacent pyrimidine bases, often two thymines (Figure 2). Approximately 80,000 pyrimidine dimers – the most common form of “photolesion” – are formed after each hour of sun exposure.1 Both replication and transcription are blocked by such dimers, presumably because the enzymes carrying out these functions cannot cope with the resulting bulge in the DNA double helix. Scientific Communiqué VI provides a detailed overview of how UVR and oxidative stress can cause DNA damage and oncogenesis.
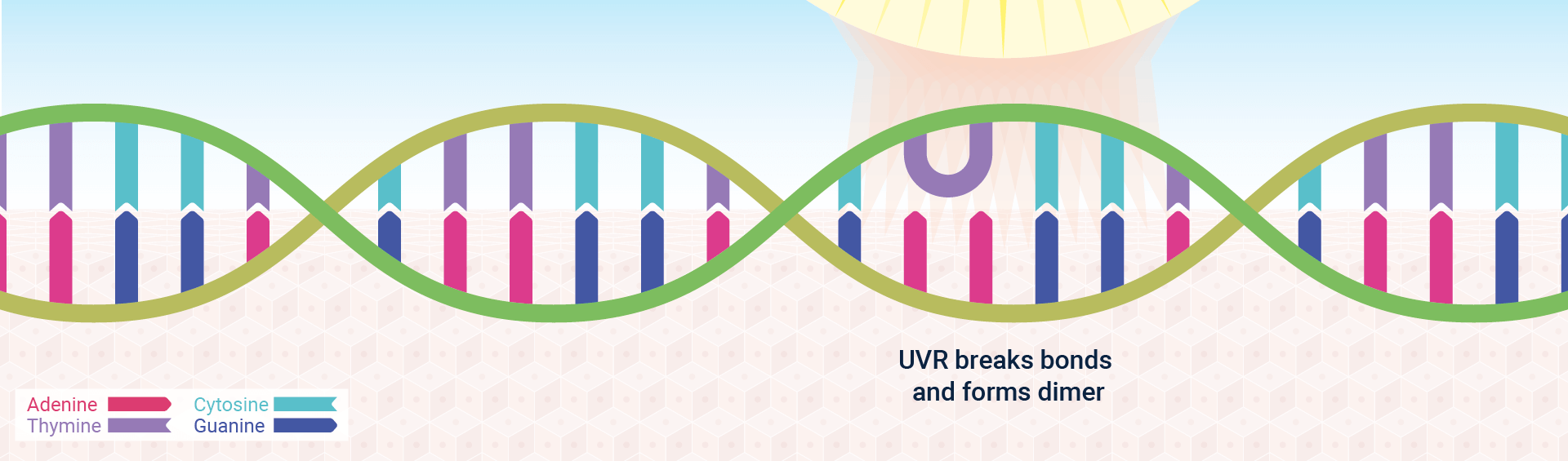

Cells have adopted a variety of strategies to repair damaged DNA, depending on the severity of the damage and whether the cell is actively undergoing cell division (and hence DNA replication).
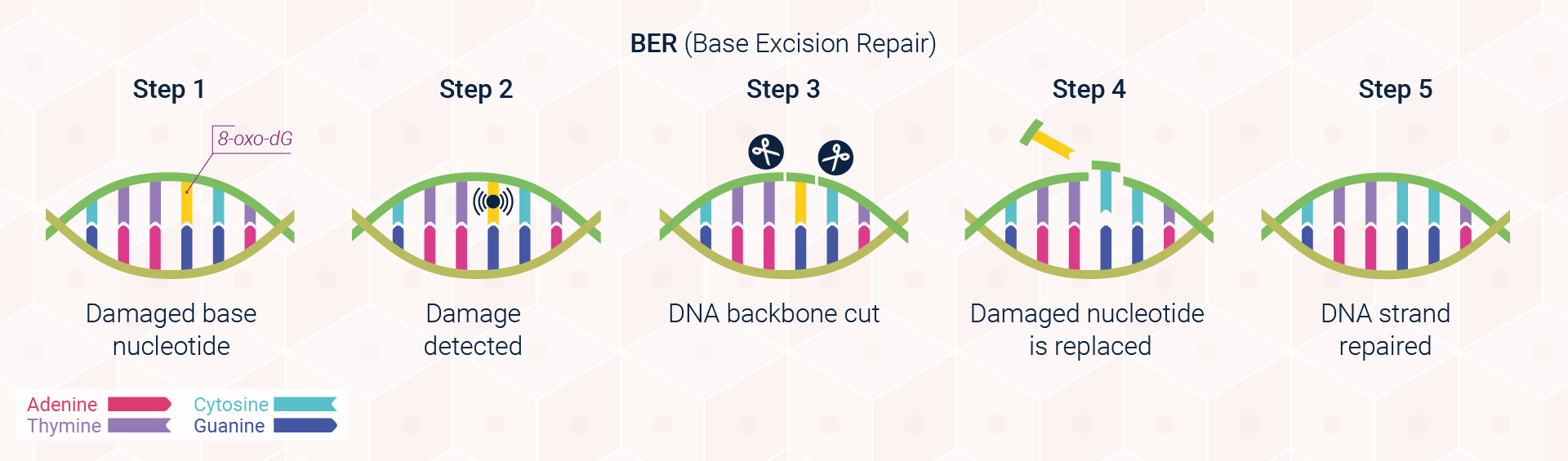

Excision repair pathways are classified into two main types: base excision repair (BER; Figure 3) and nucleotide excision repair (NER; Figure 4). The first of these pathways, BER, corrects single damaged bases in DNA. Deaminated and depurinated bases are detected by specific DNA glycosylases, which recognise a specific deaminated base and remove it from the DNA molecule by cleaving the bond between the base and the sugar to which it is attached. The sugar with the missing base is then recognised by a repair endonuclease that detects depurination called AP endonuclease. For removing pyrimidine dimers and other bulky lesions in DNA, cells employ the second type of excision repair, NER.
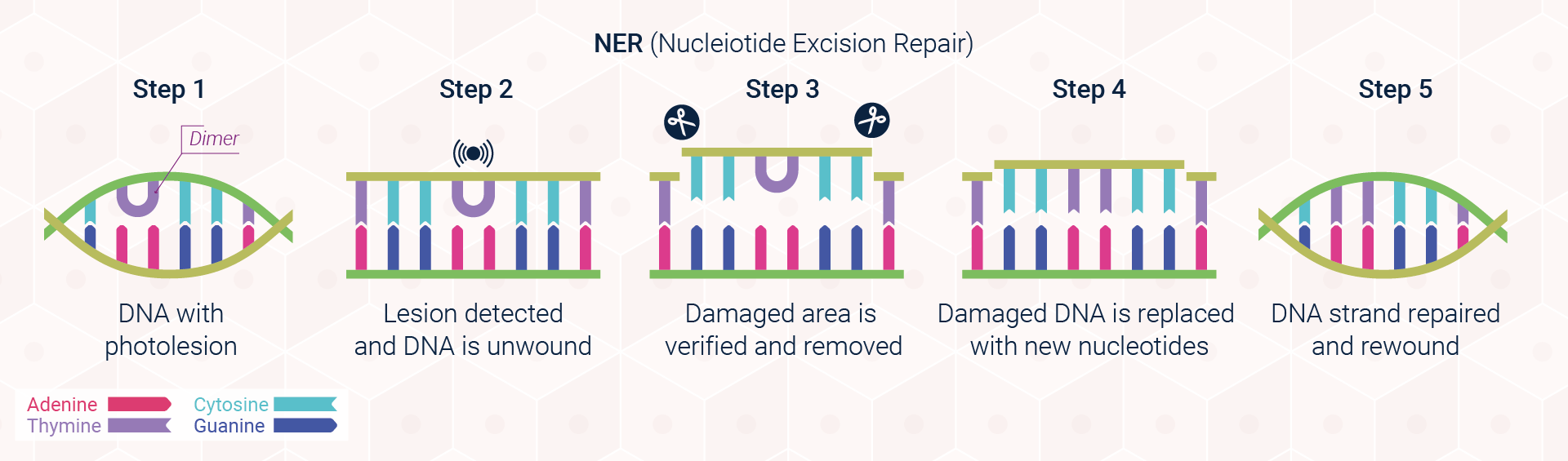

Since not all genes are transcriptionally active, however, photolesions that have been generated in the genetic sequence of dormant genes or non-coding regions of the DNA, must be identified by an alternative approach known as global genome repair (GGR). In GGR, the DNA is surveyed for any abnormalities by the UV damaging binding complex UV-DDB and XPC-RAD23B. These proteins locate, bind, and tag the DNA distortion, preparing it for removal.
In some instances where repair is not possible, however, our cells have developed an alternative strategy to cope with damage. This process, known as translesional synthesis, will be explored in further detail as part of DNA Repair Communiqué II. A more detailed view of the excision repair pathways outlined above is provided in Scientific Communiqué VIII.
The importance of NER is underscored by the plight of people who have mutations affecting the NER pathway. Individuals with XP, for example, carry a mutation in any of seven genes encoding components of the NER system. As a result, they cannot effectively repair the DNA damage caused by UVR, and thus have a considerable risk of developing skin cancer.
Xeroderma Pigmentosum: A Model of DNA Repair
For patients living with XP, exposure to the sun and UVR is extremely dangerous, causing permanent, irreparable, DNA damage and ultimately leading to a shortened life expectancy.
XP is a rare inherited disorder in which the process of DNA repair is defective, characterised by increased sensitivity to UVR, causing the early development of skin and mucous membrane cancers.
Due to the inability to initiate or complete the NER process, XP patients under 20 years of age are at 10,000–fold risk of developing non-melanoma skin cancers and 2,000–fold risk of melanoma skin cancers.2 Most XP patients will experience their first skin cancer before adolescence, with many suffering from progressive non-melanoma skin cancers and melanoma in the third decade of life. Due to the extreme rate of these malignancies, surgical intervention is frequently required.
Many of the clinical manifestations present in XP patients occur in the general population at a much older age. Patients with XP thus provide a unique model to study the impact of ageing and oncogenesis. Whereas the steps of the underlying mechanisms are not well-known, findings established from studying the XP population highlight the role for DNA repair and genomic integrity as principal factors in delaying the effects of ageing.3
Moreover, these findings are not limited to the skin. Internal manifestations of premature ageing, including peripheral neuropathy, progressive sensorineural hearing loss, and neurodegeneration, are reported in 25% of patients with XP.2 These findings – from DiGiovanna et al – highlight the versatility of the NER system in repairing damage to our DNA. Specifically, they posit how NER can remove structurally unrelated bulky lesions occurring due to endogenous factors, such as reactive oxygen species and other by-products of normal cellular metabolism.4

Concluding remarks
The stability of genetic material is fundamental to homeostasis (the maintenance of normal function). Our DNA is constantly under threat of damage from a variety of exogenous and endogenous factors, most significant of which, for our skin, is UVR. Effective DNA repair is crucial in guarding against the deleterious consequences of ageing and oncogenesis. If these repair pathways are compromised, as is the case in XP, the results can be devastating.
Thus, understanding these pathways in greater detail and providing solutions which can further reinforce and protect the fidelity of this delicate balance, should be of the utmost importance — for everyone.
Further Reading
Watson, James D., and Francis H C Crick. “Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid.” Nature 171 (1953): 737–738.
Watson, James D., and Francis H C Crick. “Genetical Implications of the Structure of Deoxyribonucleic Acid.” Nature 171 (1953): 964–967.
Meselson, Matthew, Franklin W. Stahl, and Jerome Vinograd. “Equilibrium Sedimentation of Macromolecules in Density Gradients.” Proceedings of the National Academy of Sciences 43 (1957): 581–588.
Kornberg, A. The biological synthesis of deoxyribonucleic acid. Nobel Lecture, December 11, 1959.
Preston BD, Albertson TM, Herr AJ. “DNA replication fidelity and cancer.” Semin Cancer Biol. 2010;20(5):281–293.
References
1. Benjamin, C. L., & Ananthaswamy, H. N. (2007). p53 and the pathogenesis of skin cancer. Toxicology and applied pharmacology, 224(3), 241–248.
2. DiGiovanna, J. J., & Kraemer, K. H. (2012). Shining a light on xeroderma pigmentosum. Journal of investigative dermatology, 132(3), 785–796.
3. Rizza, Elizabeth R.H, et al. “Xeroderma Pigmentosum: A Model for Human Premature Aging.” Journal of Investigative Dermatology, vol. 141, no. 4, 2021, pp. 976–984.
4. Menck, C. F., & Munford, V. (2014). DNA repair diseases: What do they tell us about cancer and aging? Genetics and Molecular Biology, 37, 220–233.
DNA Repair Communiqué Library
The DNA Repair Communiqués provide an in-depth look
at the work we conduct and the rationale for developing melanocortins.
Glossary
- AP endonuclease
- A multifunctional enzyme involved in base excision repair (BER).
- Base excision repair
- A repair mechanism that corrects DNA damage from oxidation, deamination, and alkylation.
- Cell cycle
- The series of events that take place in a cell that cause it to divide into two daughter cells.
- Chromosome
- The part of the cell that houses the genetic material.
- Chromatin
- A specialised form of DNA contained within the cell nucleus.
- Cytokinesis
- The process by which one cell physically divides into two cells.
- Deaminated base
- DNA in which an amine base has been lost (cytosine and thymine).
- Depurinated base
- DNA in which a purine base has been lost (adenine and guanine).
- DNA glycosylase
- A family of enzymes involved in base excision repair.
- DNA polymerase
- Enzyme that synthesises DNA by joining nucleotides together using a DNA template as a guide.
- Global genome repair
- A subtype of NER which repairs UVB induced lesions in the non-transcribed regions of the DNA.
- Interphase
- Interphase is composed of G1 phase (cell growth), followed by S phase, followed by G2 phase (cell growth), after which the mitotic phase begins.
- M phase
- The time-period in which the cell divides into two cells (parent and daughter cell).
- Mitosis
- A process of cell duplication, or reproduction, during which one cell gives rise to two genetically identical daughter cells.
- Mitotic spindle
- A highly dynamic molecular machine, which assembles around the chromosomes and distributes the duplicated genome to the daughter cells during mitosis.
- Nucleotide excision repair
- A process that repairs damage to one strand of the DNA, particularly from UV irradiation, which distorts the DNA helix.
- Pyrimidine dimer
- A DNA mutation formed because of UVB radiation exposure.
- S phase
- The time-period in which DNA replication occurs and the DNA molecule is duplicated.
- Semiconservative replication
- A hypothesis for cell replication wherein the DNA double helix unwinds, with each parent strand serving as a template for the synthesis of a complementary daughter strand.
- Sister chromatids
- Two identical chromatids that are formed by replication of a chromosome during the S phase of the cell cycle.
- Transcription-coupled repair
- A sub pathway of NER, it removes lesions from the template DNA strands of actively transcribed genes.
- Translesion DNA synthesis
- Process by which cells replicated damaged DNA in order to prevent blockages of the replication fork.
- Xeroderma pigmentosum
- XP is a rare autosomal recessive disorder of DNA repair characterised by one of eight defective enzymes within the Xdtgene, involved in DNA repair.