ACTH Manufacturing Update
| Melbourne, Australia, 17 March 2022 | ASX: XETRA-DAX: Level 1 ADR: |
CUV UR9 CLVLY |
Executive Summary
- NEURACTHEL® (ACTH) – part of CLINUVEL’s expanded melanocortin portfolio
- Drug substance being manufactured under current Good Manufacturing Practices
- Development and validation work in progress
- CLINUVEL translates its expertise in melanocortins and formulations
CLINUVEL is progressing scaled manufacturing of adrenocorticotropic hormone (ACTH) under current Good Manufacturing Practice (cGMP), as it strives to finalise the development and validation work necessary for a complete regulatory dossier. As part of a differentiated business strategy to develop and commercialise a broad suite of melanocortins, the Company has engaged a commercial manufacturing partner and strengthened its intellectual property portfolio.
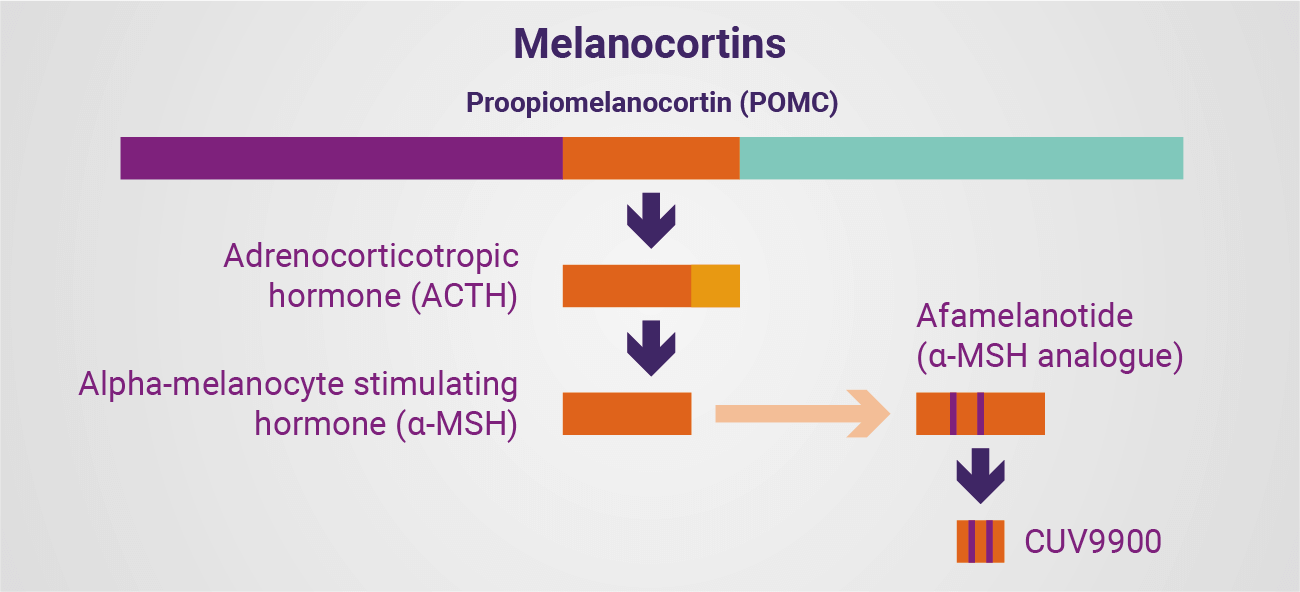
Melanocortin Family
ACTH is a human hormone, derived from the precursor peptide proopiomelanocortin (POMC), and synthesised by the pituitary gland. ACTH regulates a variety of processes, such as energy homeostasis and metabolism, cortisol production, and nerve regeneration. An injectable gel formulation of ACTH is approved by the US Food and Drug Administration (FDA) for the treatment of 19 different medical conditions, including infantile spasms, acute exacerbations of multiple sclerosis, and rheumatic disorders.
CLINUVEL has identified broader clinical potential for ACTH, a hormone to treat neurological, endocrinological, and degenerative disorders, and announced in November 2021 that it was adding ACTH to its existing melanocortin portfolio.

Global Opportunity for NEURACTHEL®
CLINUVEL will launch its ACTH product range under the trade names NEURACTHEL® Instant and NEURACTHEL® Modified-release for patients with neurological, endocrinological, and degenerative disorders.
Worldwide, the ACTH market has been growing annually since 2014, with a projected CAGR of 3.9% from 2021 to 2031. The global use of ACTH was valued at US$1.29 billion in 2020, and projected to reach US$1.91 billion by 2031.¹ The driving factors for the expansion of the global ACTH market include the rise in the number of diseases and patients to be diagnosed and treated by the bioactive hormone.
Commentary
“We are differentiating CLINUVEL from any other pharmaceutical group by manufacturing and developing the further use of NEURACTHEL®,” CLINUVEL’s Vice President of Scientific Affairs, Dr Tim Zhao said. “Not only are we focusing our knowledge on this family of bioactive peptides, but also widening the scope of use for ACTH, a potent hormone.
”We have a clear vision of what we are building towards and how to reach many patients in need. The huge advantage of our program is already the cumulative clinical exposure and experiences of this hormone in different regions and patient groups, such that there is available indicative safety and efficacy information from which we can build and derive novelty,” Dr Zhao said.
– End –
- Adrenocorticotropic hormone (ACTH) market – global industry analysis, size, share, trends, and forecast, 2017-2031 by Transparency Market Research (TMR), 2021.
About Adrenocorticotropic Hormone (ACTH)
ACTH is a naturally occurring hormone which plays an important role in the production of cortisol, enabling the combat of stress and regulation of immune responses, maintenance of blood pressure, moderation of blood sugar, and regulation of metabolism.
Developed as a therapeutic agent in the 1950s, ACTH was first administered for human use as an animal derived hormone to influence the glucocorticoid secretion from the adrenal glands, and to treat a host of neurological and inflammatory diseases.
Pharmaceutical products containing ACTH or its analogues – including Cortrosyn®, Synacthen®, and Acthar® – are licensed in the USA, Europe and Asia-Pacific region in different dosage forms, including a solution with instant-release characteristics and a slow-release gel. ACTH analogues in liquid and gel formulations are used in severe chronic and acute neurological, endocrinological, and degenerative disorders. Cortrosyn®, Synacthen® and Acthar® are trademarks of their respective owners.
About CLINUVEL PHARMACEUTICALS LIMITED
CLINUVEL (ASX: CUV; ADR LEVEL 1: CLVLY; XETRA-DAX: UR9) is a global specialty pharmaceutical group focused on developing and commercialising treatments for patients with genetic, metabolic, systemic, and life-threatening, acute disorders, as well as healthcare solutions for the general population. As pioneers in photomedicine and the family of melanocortin peptides, CLINUVEL’s research and development has led to innovative treatments for patient populations with a clinical need for systemic photoprotection, DNA repair, repigmentation and acute or life-threatening conditions who lack alternatives.
CLINUVEL’s lead therapy, SCENESSE® (afamelanotide 16mg), is approved for commercial distribution in Europe, the USA, Israel and Australia as the world’s first systemic photoprotective drug for the prevention of phototoxicity (anaphylactoid reactions and burns) in adult patients with erythropoietic protoporphyria (EPP). Headquartered in Melbourne, Australia, CLINUVEL has operations in Europe, Singapore and the USA. For more information, please go to https://www.clinuvel.com.
SCENESSE®, PRÉNUMBRA®, and NEURACTHEL® are registered trademarks of CLINUVEL.
Authorised for ASX release by the Board of Directors of CLINUVEL PHARMACEUTICALS LTD
Media Enquiries
Monsoon Communications
Mr Rudi Michelson, 61 411 402 737, rudim@monsoon.com.au
Head of Investor Relations
Mr Malcolm Bull, CLINUVEL PHARMACEUTICALS LTD
Investor Enquiries
https://www.clinuvel.com/investors/contact-us
Forward-Looking Statements
This release contains forward-looking statements, which reflect the current beliefs and expectations of CLINUVEL’s management. Statements may involve a number of known and unknown risks that could cause our future results, performance or achievements to differ significantly from those expressed or implied by such forward-looking statements. Important factors that could cause or contribute to such differences include risks relating to: our ability to develop and commercialise pharmaceutical products; the COVID-19 pandemic and/or other world, regional or national events affecting the supply chain for a protracted period of time, including our ability to develop, manufacture, market and sell biopharmaceutical products; competition for our products, especially SCENESSE® (afamelanotide 16mg), PRÉNUMBRA® or NEURACTHEL®; our ability to achieve expected safety and efficacy results in a timely manner through our innovative R&D efforts; the effectiveness of our patents and other protections for innovative products, particularly in view of national and regional variations in patent laws; our potential exposure to product liability claims to the extent not covered by insurance; increased government scrutiny in either Australia, the U.S., Europe, Israel, China and Japan of our agreements with third parties and suppliers; our exposure to currency fluctuations and restrictions as well as credit risks; the effects of reforms in healthcare regulation and pharmaceutical pricing and reimbursement; that the Company may incur unexpected delays in the outsourced manufacturing of SCENESSE®, PRÉNUMBRA® or NEURACTHEL® which may lead to it being unable to supply its commercial markets and/or clinical trial programs; any failures to comply with any government payment system (i.e. Medicare) reporting and payment obligations; uncertainties surrounding the legislative and regulatory pathways for the registration and approval of biotechnology and consumer based products; decisions by regulatory authorities regarding approval of our products as well as their decisions regarding label claims; our ability to retain or attract key personnel and managerial talent; the impact of broader change within the pharmaceutical industry and related industries; potential changes to tax liabilities or legislation; environmental risks; and other factors that have been discussed in our 2021 Annual Report. Forward-looking statements speak only as of the date on which they are made, and the Company undertakes no obligation, outside of those required under applicable laws or relevant listing rules of the Australian Securities Exchange, to update or revise any forward-looking statement, whether as a result of new information, future events or otherwise. More information on preliminary and uncertain forecasts and estimates is available on request, whereby it is stated that past performance is not an indicator of future performance.
www.clinuvel.com
Level 11, 535 Bourke Street, Melbourne, Victoria, Australia, 3000, T +61 3 9660 4900, F +61 3 9660 4909