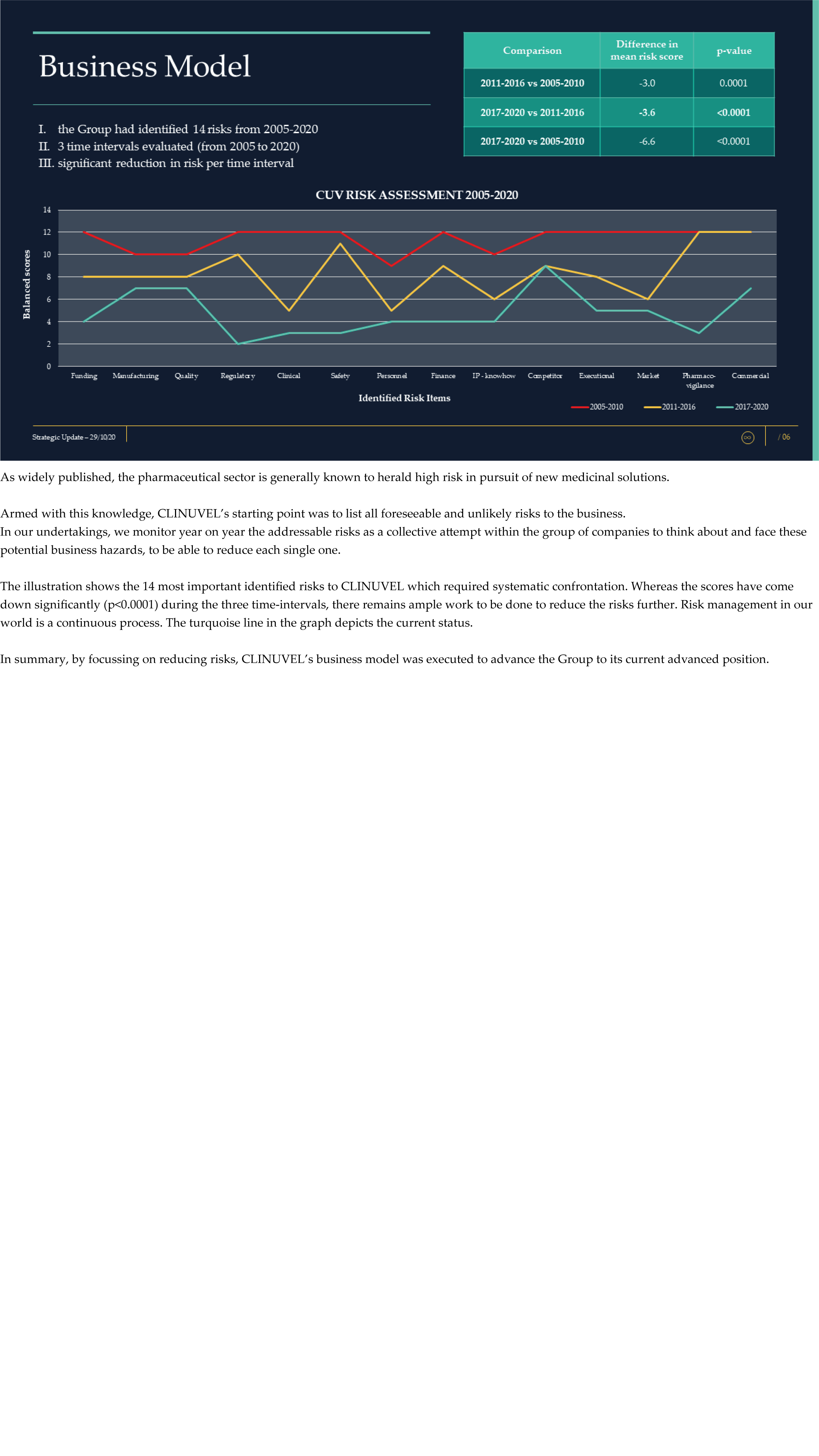
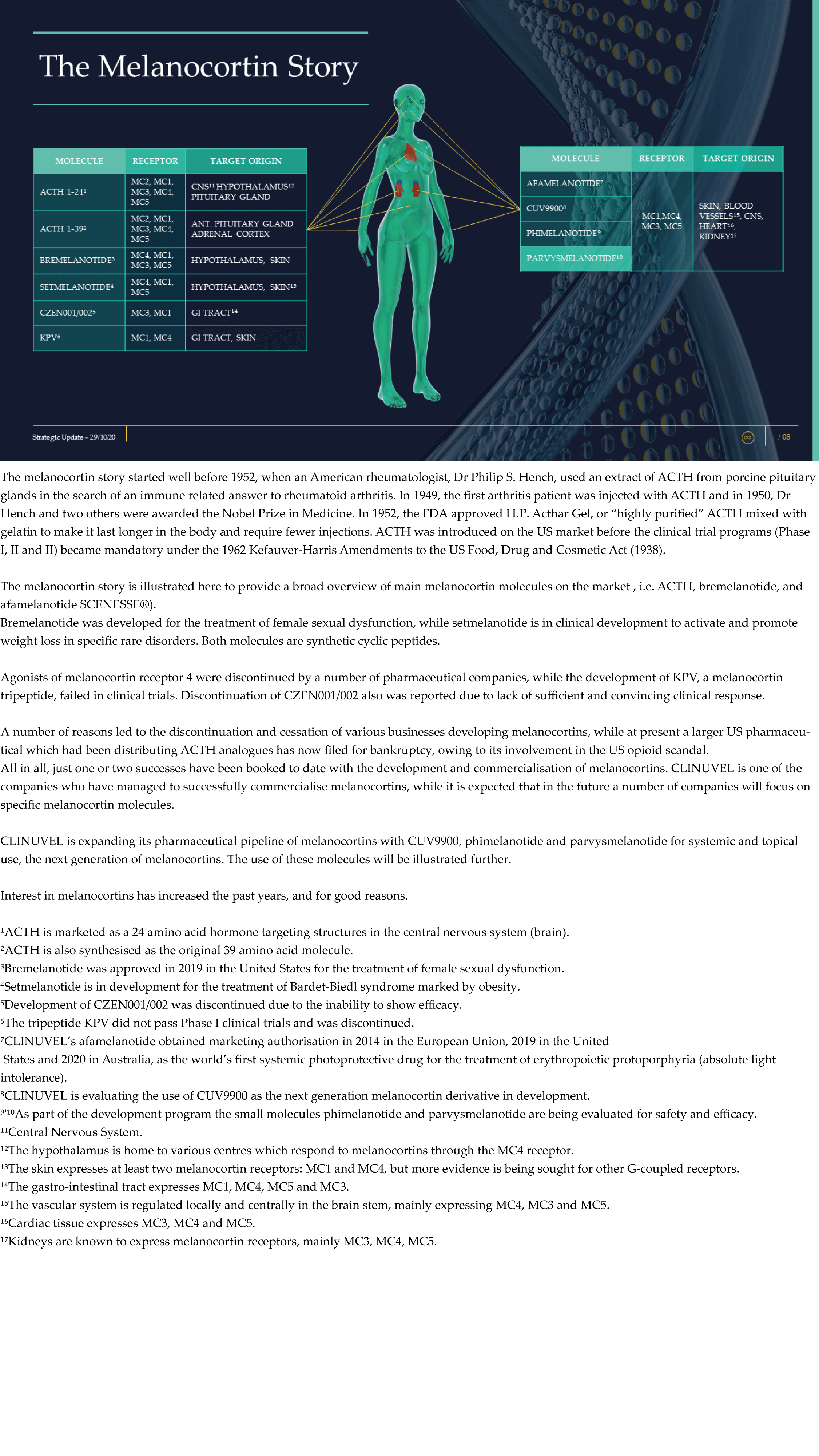
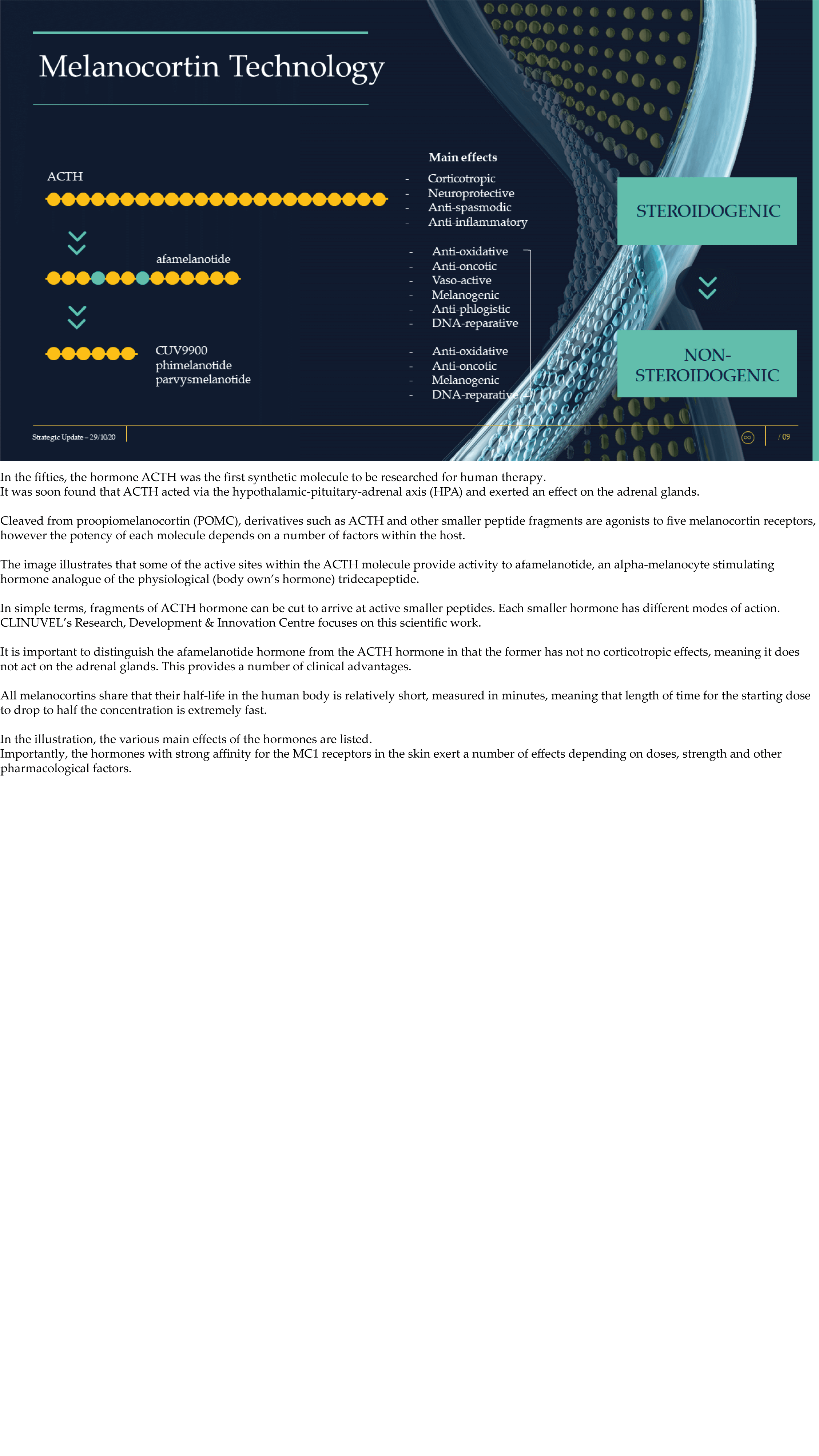
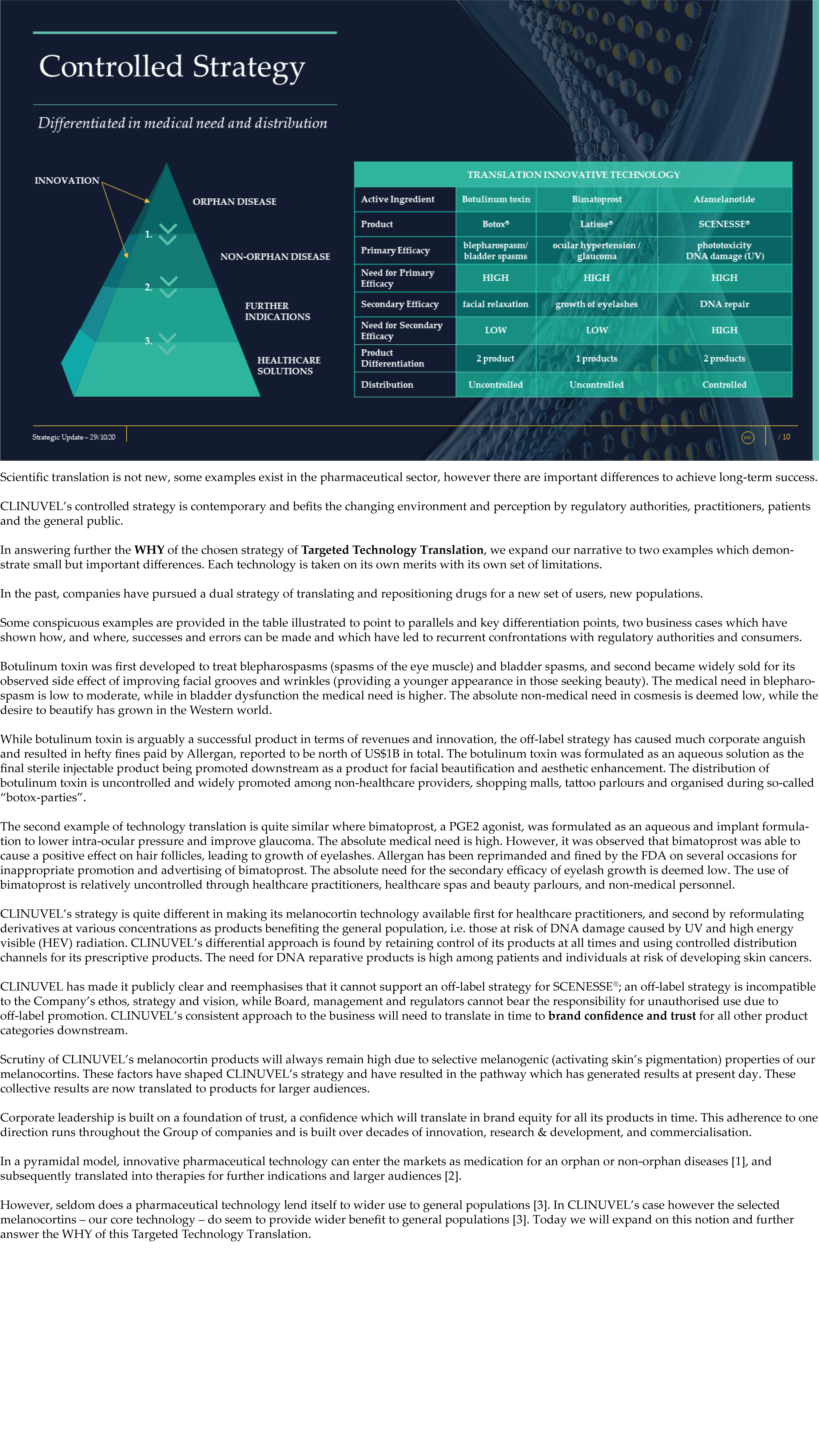
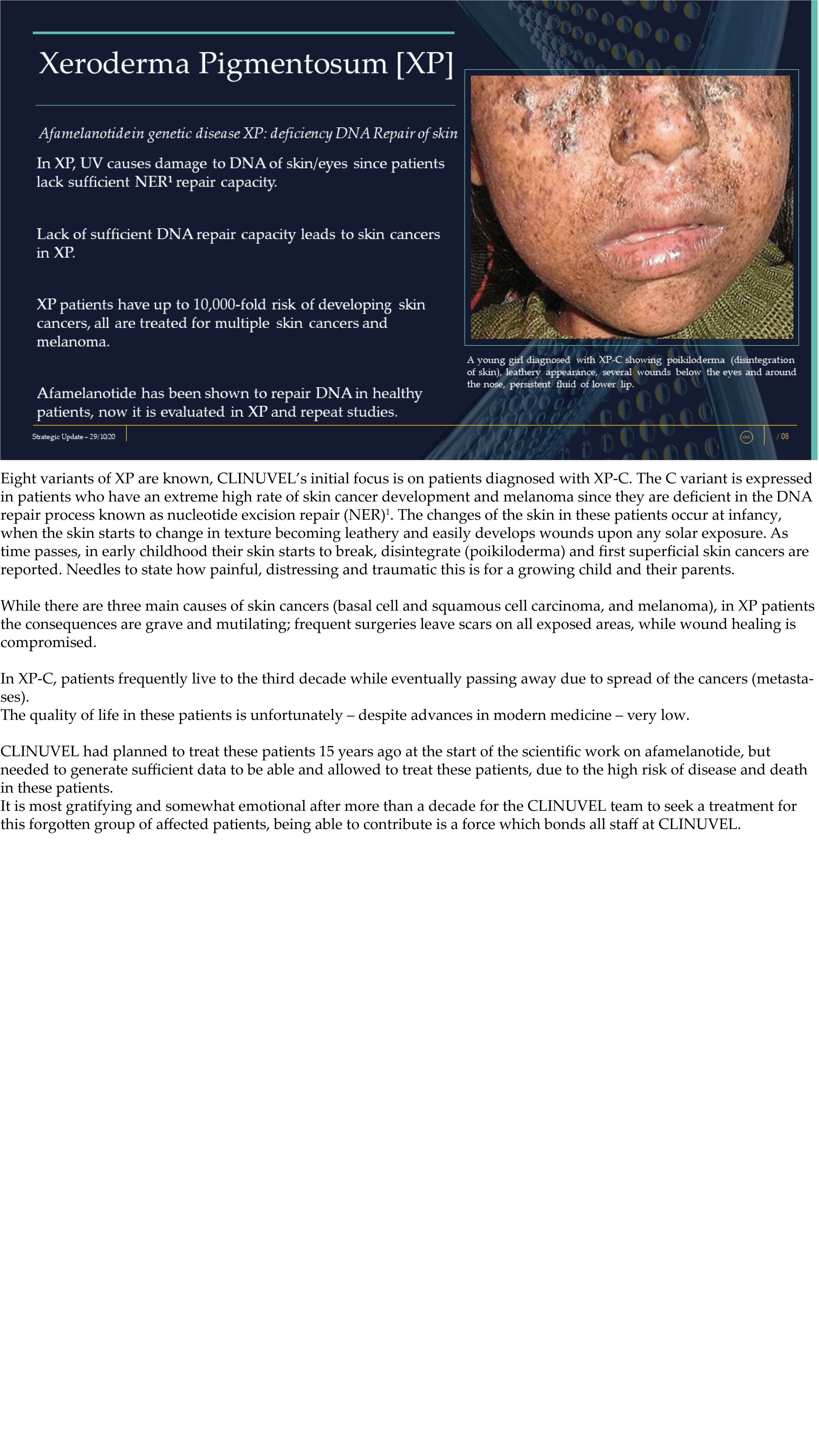
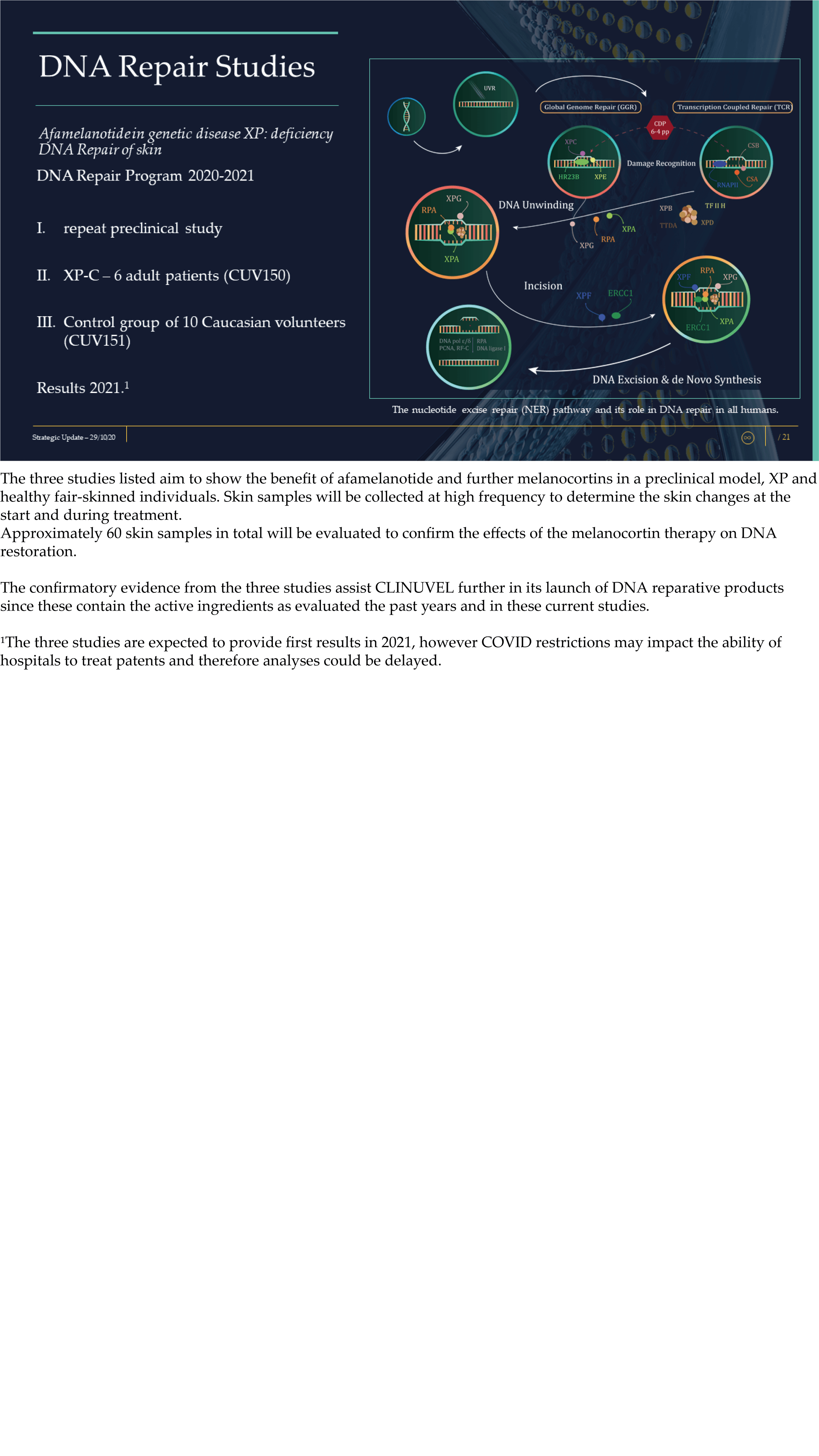
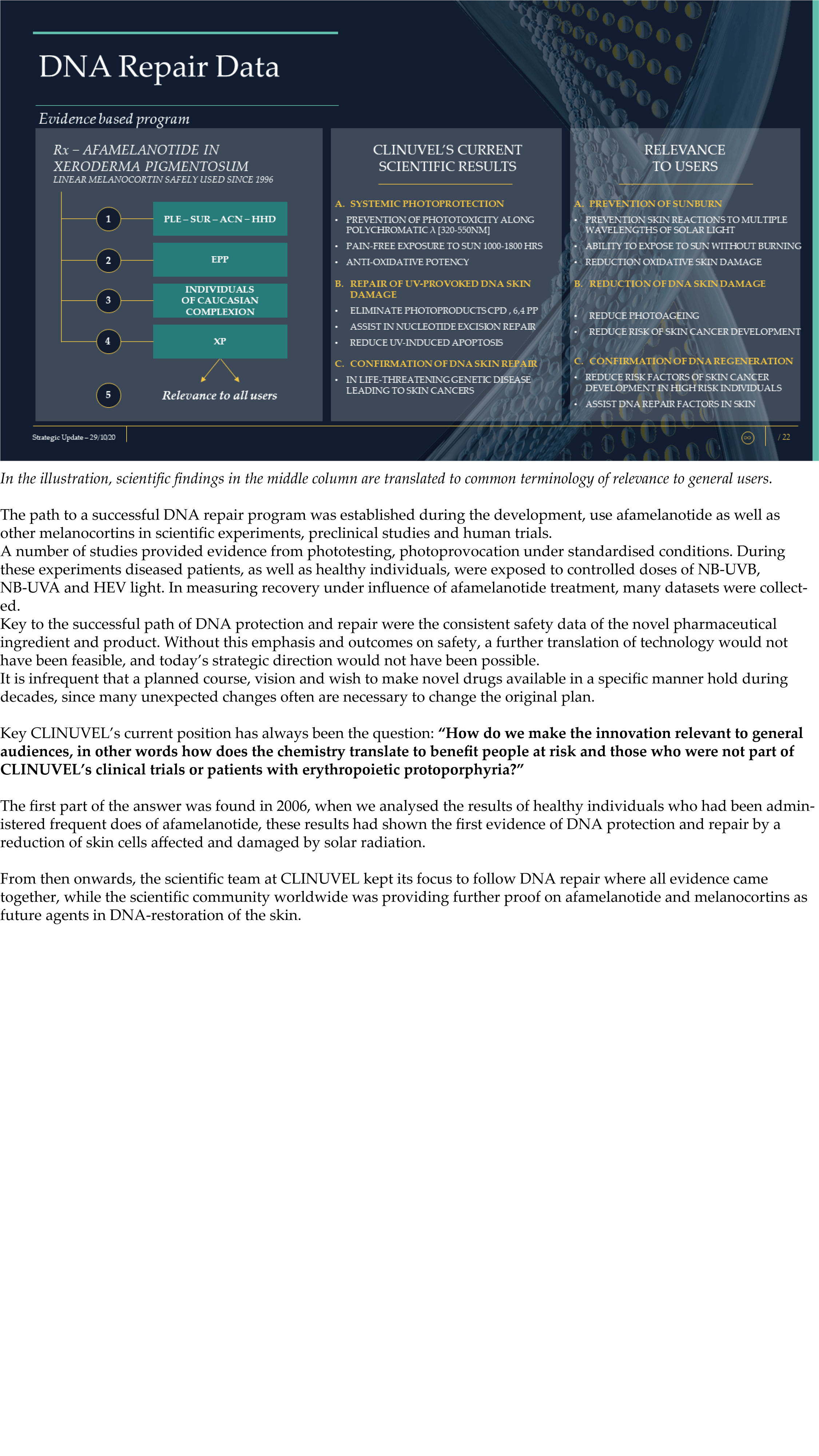
CLINUVEL Strategic Update – Extended
CLINUVEL PHARMACEUTICALS LTD today released a strategic update on its business. An extensive version (31 slide illustrations) and executive summary (13 slide illustrations) have both been lodged with the ASX.
CLINUVEL is focussing on the commercialisation of the medicinal product SCENESSE® (afamelanotide controlled-release) for the treatment of erythropoietic protoporphyria (EPP) in the European Union, United States and – since 26 October – Australia.
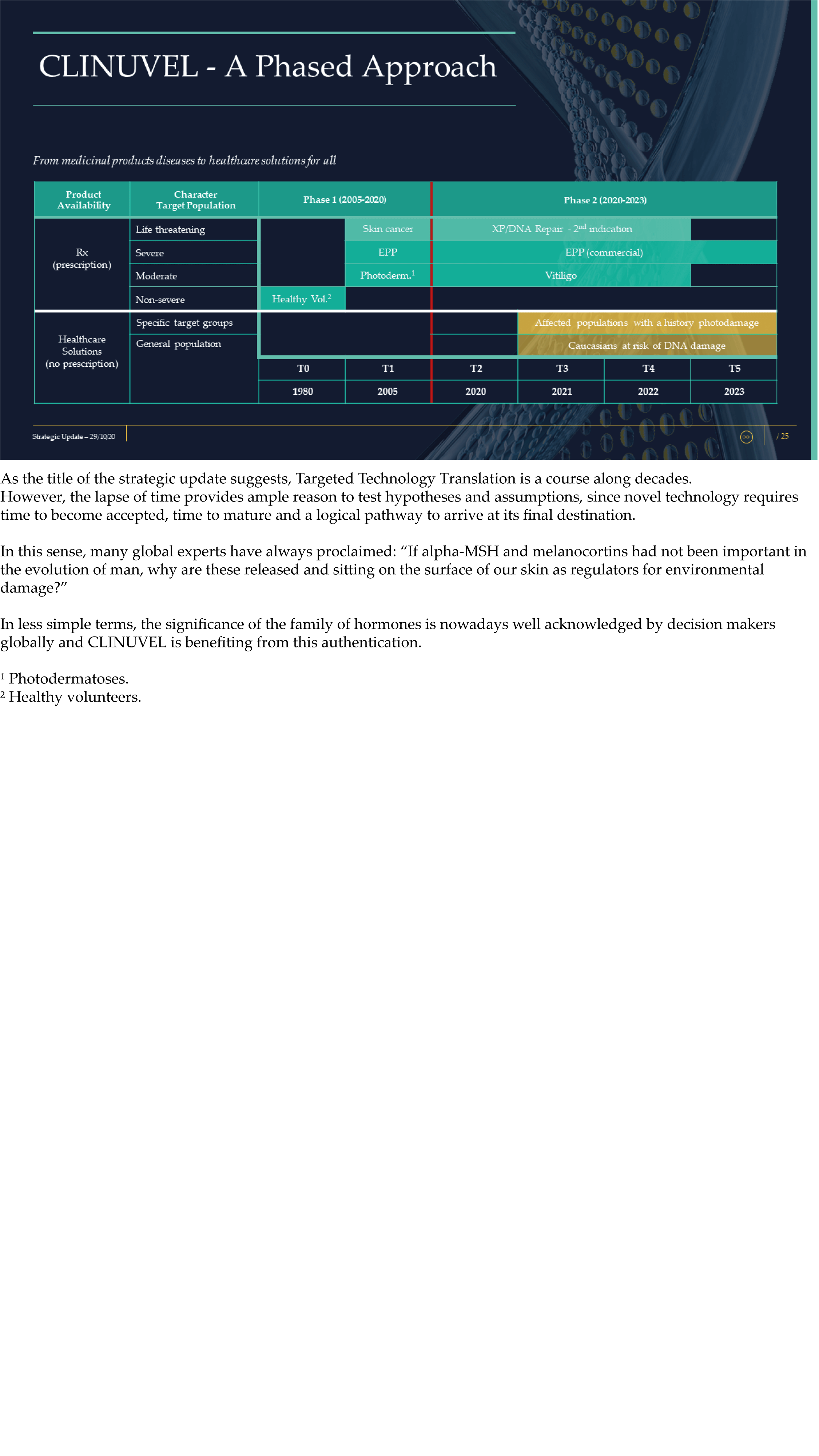
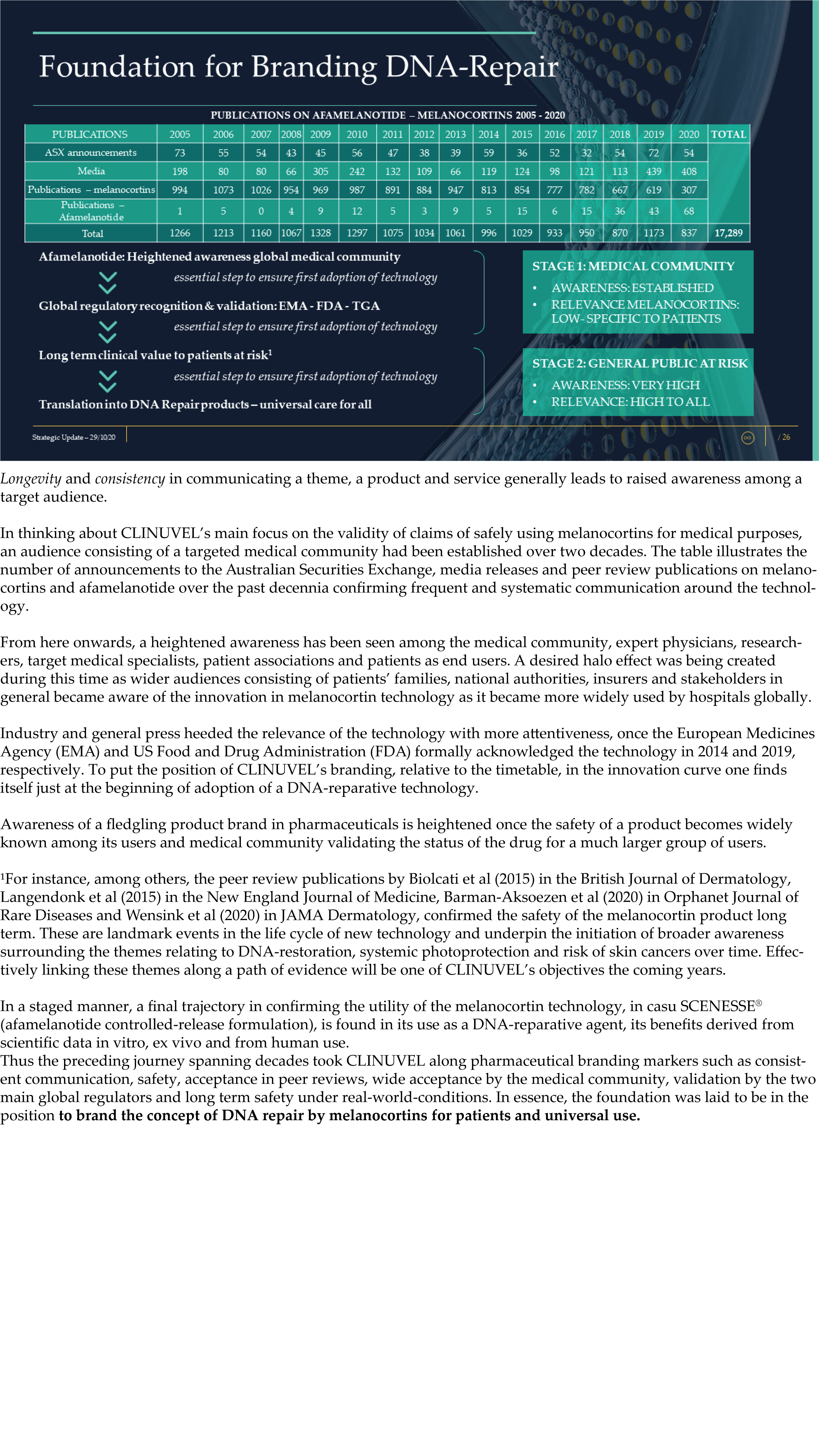
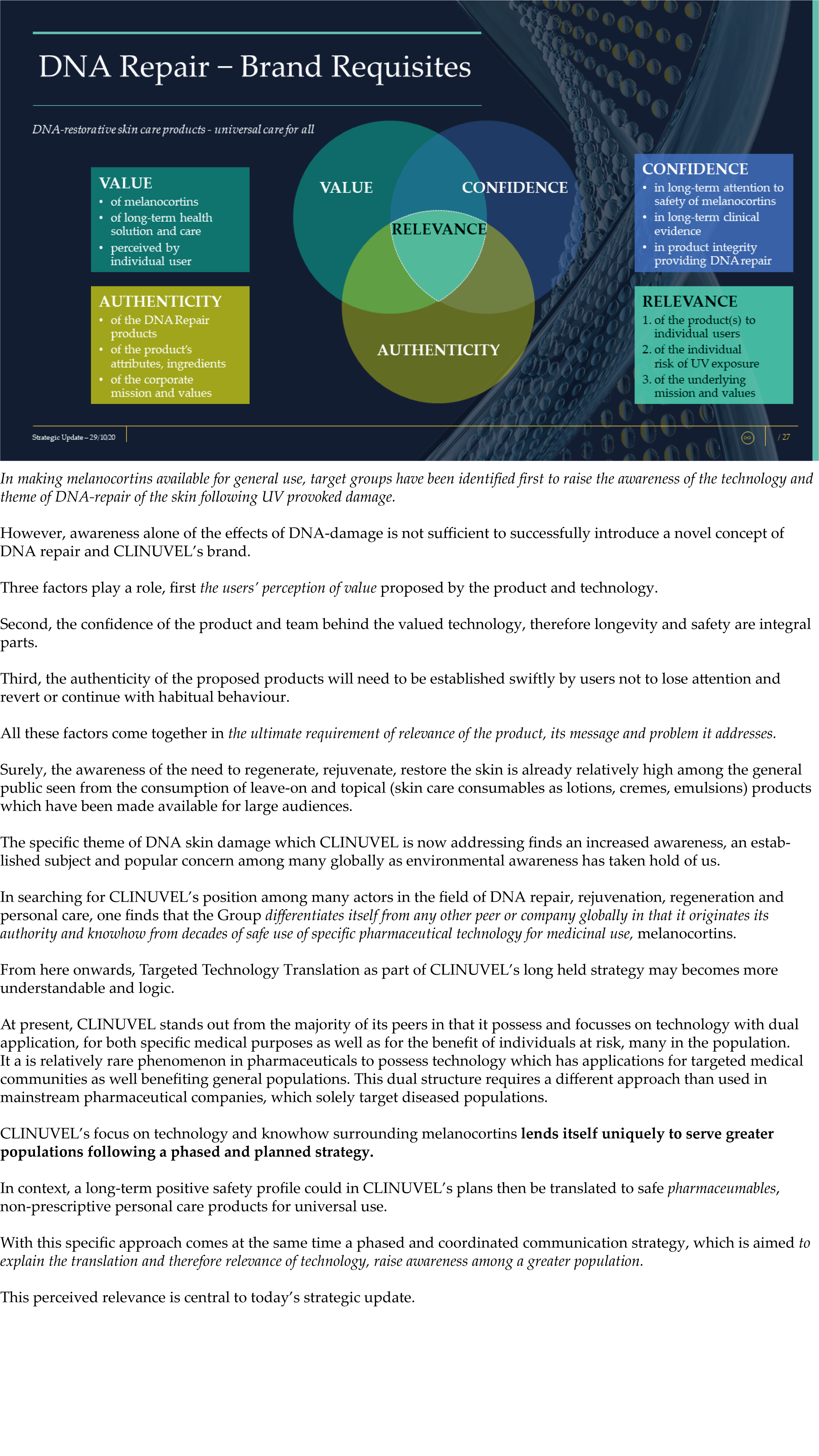
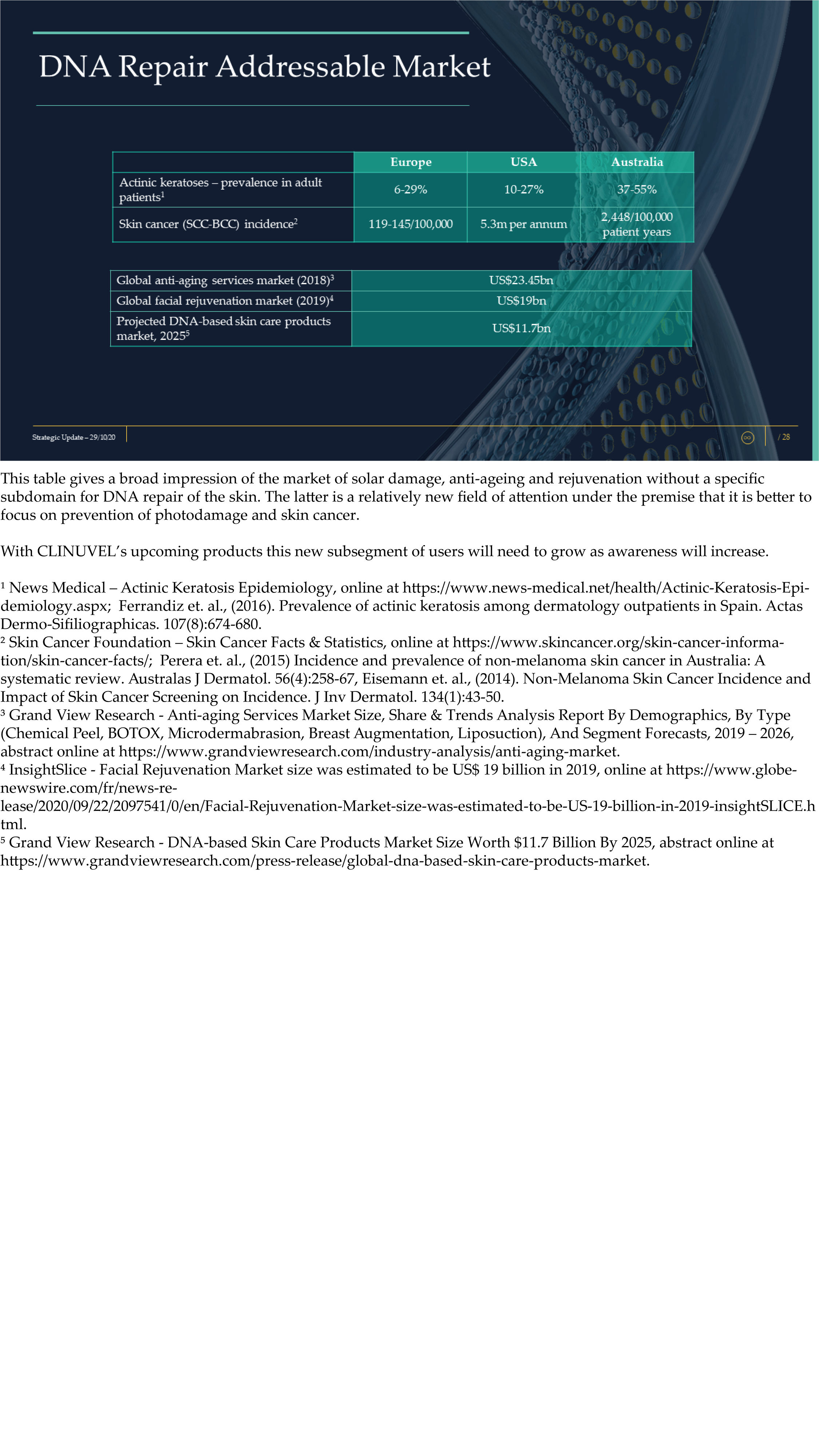
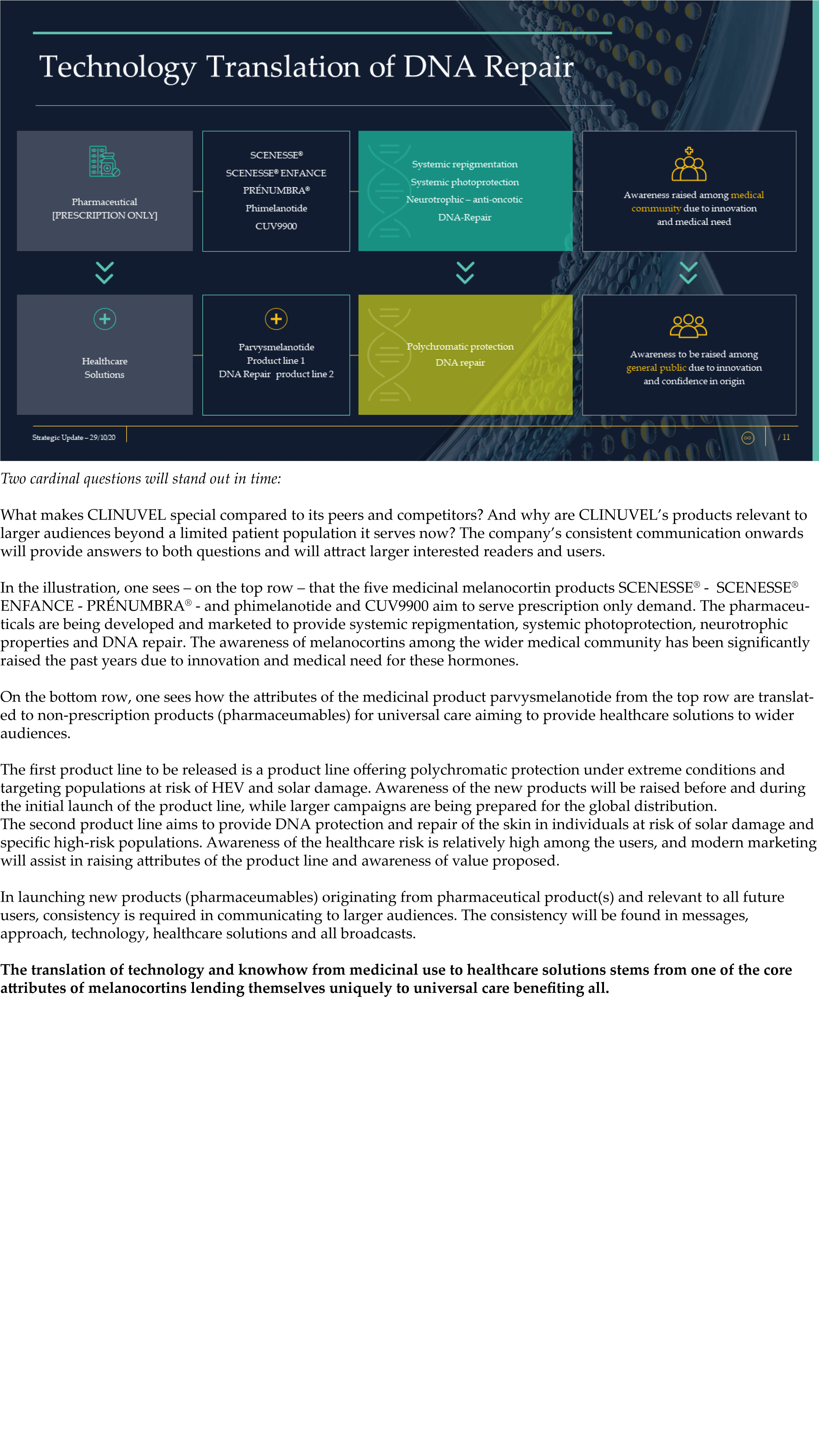
The Update reveals CLINUVEL’s strategic intentions are described as dual: ongoing work to scientifically translate melanocortins as prescription medicines for further life-threatening disorders as well as making the technology available for healthcare solutions as non-prescription products. The focus of the latter product category is to provide DNA protection and repair of the skin in individuals at highest risk of solar skin damage from UV exposure.















Previous
Next
Download the presentation here