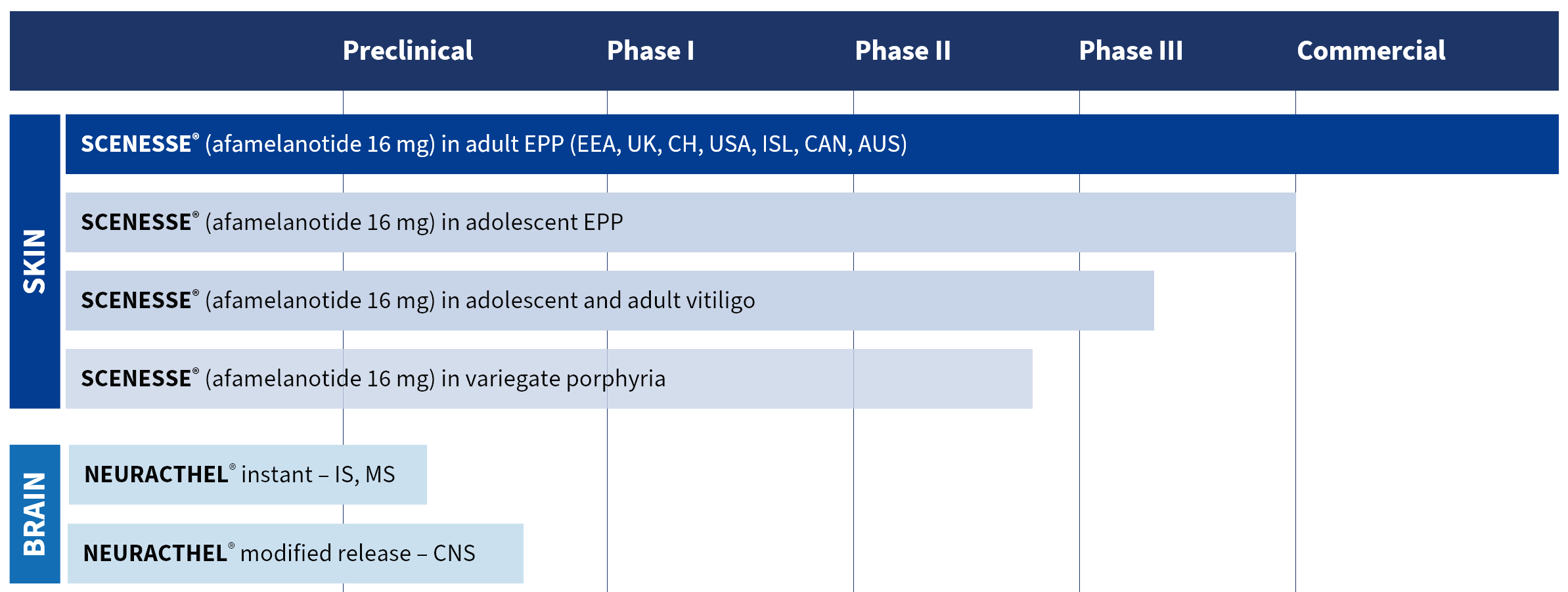
Pharmaceutical development
CLINUVEL is a global specialty pharmaceutical group focused on developing and commercialising treatments for patients with genetic, metabolic, systemic, and life-threatening, acute disorders. We work to translate scientific concepts into commercial products for patients with unmet needs and the general population.
Published research
McNeil, M. M., Nahhas, A. F., Braunberger, T. L., Hamzavi, I. H. Afamelanotide in the Treatment of Dermatologic Disease. Skin Ther Lett. 2018;23:6-10.
Rodrigues, M., Ezzedine, K., Hamzavi, I., Pandya, A. G., Harris, J. E., Vitiligo Working Group. Current and emerging treatments for vitiligo. Journal of the American Academy of Dermatology. 2017;77(1):17-29.
Minder, El, Barman-Aksoezen, J, Schneider-Yin, X. Pharmacokinetics and pharmacodynamics of afamelanotide and its clinical use in treating dermatologic disorders. Clinical Pharmacokinetics. 2017;56(8):815-823.
Lane, AM, McKay, JT, Bonkovsky, HL. Advances in the management of erythropoietic protoporphyria–role of afamelanotide. The application of clinical genetics. 2016;9:179.
Minder E, Schneider-Yin X. Afamelanotide (CUV1647) in dermal phototoxicity of erythropoietic protoporphyria. Expert review of clinical pharmacology. 2015;8(1):43-53.
Lim HW, Grimes PE, Agbai O, et al. Afamelanotide and Narrowband UV-B Phototherapy for the Treatment of Vitiligo. JAMA dermatology. 2015;151(1):42-50.
Langendonk J, Balwani M, Anderson K et al. Afamelanotide for Erythropoietic Protoporphria. New England Journal of Medicine. 2015;373(1):48-59.
Biolcati G, Marchesini E, Sorge F, Barbieri L, Schneider‐Yin X, Minder E. Long-term observational study of afamelanotide in 115 patients with erythropoietic protoporphyria. British Journal of Dermatology. 2015;172(6):1601-1612.
Bohm M, Ehrchen J, Luger TA. Beneficial effects of the melanocortin analogue Nle(4) -d-Phe(7) -α-MSH in acne vulgaris. Journal of the European Academy of Dermatology and Venereology. 2014;28(1):108-111.
Biolcati G, Aurizi C, Barbieri L, Cialfi S, Screpanti I, Talora C. Efficacy of the melanocortin analogue Nle4-D-Phe7-α-melanocyte-stimulating hormone in the treatment of patients with Hailey–Hailey disease. Clinical and experimental dermatology. 2014;39(2):168-175.
Fabrikant J, Touloei K, Brown SM. A Review and Update on Melanocyte Stimulating Hormone Therapy: Afamelanotide. Journal of drugs in dermatology: JDD. 2013;12(7):775.
Haylett AK, Nie Z, Brownrigg M, Taylor R, Rhodes L. Systemic photoprotection in solar urticaria with α-melanocyte stimulating hormone analogue [Nle(4) -D-Phe(7) ]-α-MSH. British Journal of Dermatology. 2011;164(2):407-414.
Minder, EI. Afamelanotide melanocortin MC1 receptor agonist photoprotective agent. Drugs of the Future. 2010;35(5):365-372.
Harms, J, Lautenschlager S, Minder CE, Minder EI. An α-melanocyte–stimulating hormone analogue in erythropoietic protoporphyria. New England Journal of Medicine. 2009;360(3):306-307.
Levine N, Dorr R, Ertl G, Brooks C, Alberts D. Effects of a potent synthetic melanotropin, Nle4-D-Phe7-α-MSH (Melanotan-I) on tanning: a dose-ranging study. Journal of dermatological treatment. 1999;10(2):127-132.
Hadley ME, Dorr RT. Melanocortin peptide therapeutics: historical milestones, clinical studies and commercialization. Peptides. 2006;27(4):921-930.
Fitzgerald LM, Fryer JL, Dwyer T, Humphrey SM. Effect of MELANOTAN®,[Nle4, D-Phe7]-α-MSH, on melanin synthesis in humans with MC1R variant alleles. Peptides. 2006;27(2):388-394.